November 11, 2013 — Corindus Vascular
Robotics presented a live percutaneous coronary intervention (PCI) during the Transcatheter Cardiovascular Therapeutics (TCT) conference on Oct. 29. The procedure, led by Giora Weisz, M.D., director of clinical research, Center for Interventional Vascular Therapy, Columbia University Medical Center, was transmitted live to more than 1,000 attendees of TCT at the Moscone Center in San Francisco, Calif.
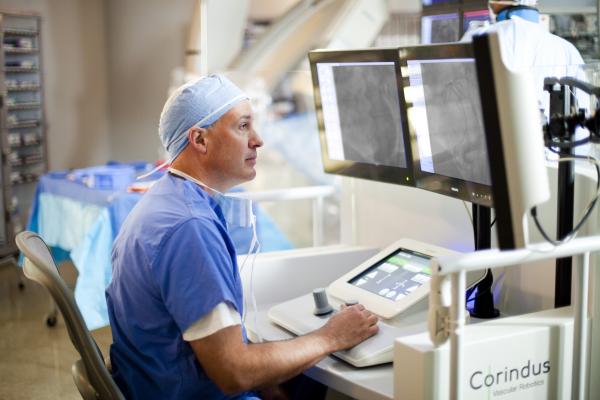
The procedure was performed using the CorPath System, the first medical device to provide robotic precision and accuracy to coronary angioplasty to help optimize clinical outcomes. During the procedure, Weisz was seated in CorPath’s interventional cockpit, remotely controlling the advancement and placement of the balloon/stent
catheter with exact, calculated movements. Using the system’s measurement function to select the proper length stent and place it in a blocked coronary artery, Weisz was precisely control the stent advancement. This particular case involved a patient with multiple risk factors and severe coronary artery disease (CAD) with multiple interventions.
“Robotic technology is changing the way interventional cardiologists conduct PCIs,” said Weisz. “I have performed more than 70 procedures using the CorPath and firmly believe in its potential to improve patient outcomes and provide a new standard of care in coronary angioplasty procedures. Presenting the case in real-time serves as an educational tool for interventional cardiologists and an example of how to provide clinical applicability, utility and safety during a robotic PCI.”
The CorPath System is the first and only U.S. Food and Drug Administration (FDA)-approved technology that enables precise, robotic-assisted angioplasties to open arteries and restore blood flow in patients with coronary artery disease. During a CorPath procedure, the interventional cardiologist sits in the radiation shielded interventional cockpit and advances stents and guidewires via a joystick with millimeter-by-millimeter robotic precision. CorPath may improve clinical outcomes by enabling precise measurement of the anatomy, which could potentially lead to better stent placements.
“Working with Dr. Weisz and other global leaders in interventional cardiology procedures and science, Corindus is bringing unprecedented robotic precision to coronary angioplasty procedures to help optimize clinical outcomes and minimize associated costs,” said David Handler, president and CEO, Corindus Vascular Robotics. “By utilizing one stent per lesion, hospitals can minimize readmissions and other costs associated with improper stent placement.”
For more information: www.corindus.com