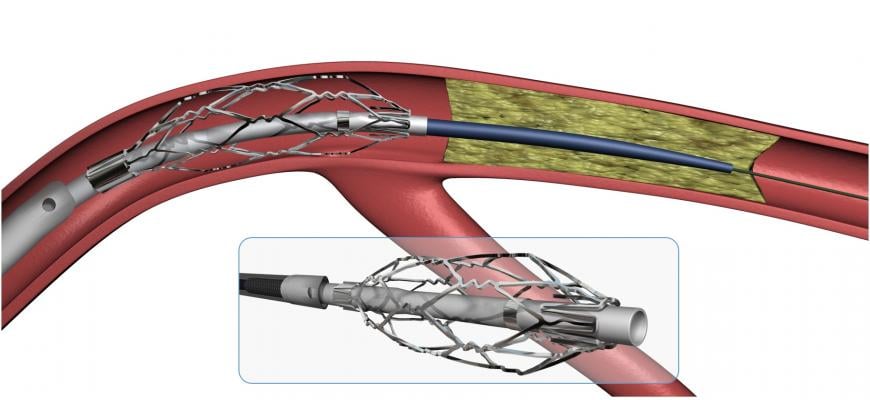
August 9, 2017 — Roxwood Medical Inc. recently announced it has entered into an exclusive agreement with Abbott for distribution of Roxwood products in the United States.
Roxwood's anchoring catheters (CenterCross Ultra, MultiCross) and microcatheters (MicroCross) offer minimally invasive platforms for physicians to percutaneously treat patients with chronic blockages by facilitating guidewire access across the blockage, often times averting an invasive bypass procedure. Roxwood's anchoring and microcatheters will join Abbott's other product offerings including the Xience drug-eluting stent and the Optis integrated optical coherence tomography system.
Percutaneous coronary interventions (PCI) are considered complex when patients have other chronic conditions such as diabetes or advanced kidney disease, when multiple vessels are involved, or if a vessel is severely blocked. The majority of PCIs performed today in the United States are considered complex in nature.
For more information: www.roxwoodmedical.com


 November 14, 2025
November 14, 2025 









