Hydra Transcatheter Aortic Heart Valve
February 14, 2022 — SMT (Sahajanand Medical Technologies Limited) has announced the publication of Hydra Transcatheter Aortic Heart Valve CE study data in the Journal of American College of Cardiology Cardiovascular Interventions journal. This study was evaluating 30-days and 1-year safety and performance of the Hydra transcatheter aortic heart valve in the treatment of symptomatic severe aortic stenosis in cardiac patients at high or extremely high surgical risk.
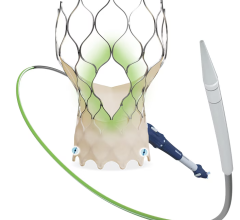
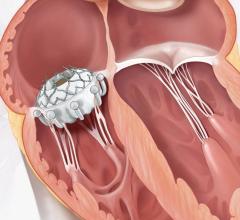
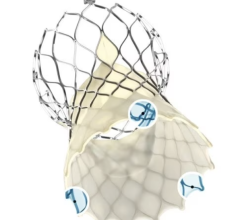
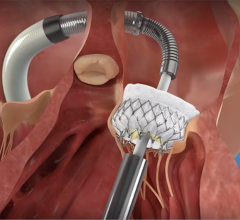
The Hydra Valve system is approved in Europe (CE mark) and several other countries in Asia to treat people with symptomatic, severe aortic stenosis who are at high or extreme risk for open-heart surgery. Aortic stenosis is one of the most common and life-threatening heart valve diseases. It occurs when the aortic valve's opening narrows and restricts blood flow from the left ventricle to the aorta. Patients with the disease can experience breathlessness, chest pressure or tightness, fainting, palpitations, fatigue, and heart murmurs. The condition can ultimately lead to heart failure. While many people don't have noticeable symptoms, more than one in eight aged 75 and older has moderate or severe aortic stenosis, which reduces the heart's pumping ability. Prior to TAVR, the standard of care for severe aortic stenosis was surgical aortic valve replacement, but not all patients were candidates for open-heart surgery. The Hydra Valve system provides a robust solution due to its flexible delivery system and supra annular leaflets and it can specifically become a good choice for patients who may have:
- the risk of patient prosthesis mismatch
- the risk of new conduction abnormalities
- a tortuous anatomy including horizontal aorta due to flexible delivery system
- or a need for future coronary interventions
In early 2021, the Genesis study on safety and performance of Hydra valve system which included 40 patients across 11 centres of India was published in the journal Catheterization and Cardiovascular Interventions.
The “30-Days and 1-year Outcomes of TAVI with the novel Hydra THV – The Hydra CE study” published now in JACC: Cardiovascular Interventions is again a multicentre study conducted across 18 medical centres in European and Asia-Pacific countries led by Prof. Dr. Lars Sondergaard from Rigshospitalet in Copenhagen, Denmark which included as many as 157 patients with a mean age of 80 years.
The Hydra CE study has demonstrated that transcatheter aortic valve replacement with Hydra valve offered favorable efficacy at 1 year, providing a large effective orifice area and low transvalvular gradient as well as acceptable complication rates.
Elaborating more about the findings of this study, Prof. Dr. Lars Sondergaard said, “The Hydra THV system is designed to overcome some of the limitations of existing devices for TAVI. The delivery system has a low profile and is very flexible, which allows for transfemoral access even in challenging anatomies. Furthermore, the THV is based on self-expanding technology with supra-annular leaflet position, which provides optimal hemodynamic performance. And the stent frame design with large cells allows for easy access to the coronary arteries, and the part in the left ventricle outflow tract has little interference with the conduction system. In addition, it is possible to re-sheath and re-position the THV, which allows for more accurate deployment. Therefore, the Hydra THV system offers several advantages compared to existing systems.”
References:
1. https://www.jacc.org/doi/abs/10.1016/j.jcin.2021.09.004
2. https://onlinelibrary.wiley.com/doi/10.1002/ccd.29733
For more information: https://smtpl.com/


 July 08, 2024
July 08, 2024