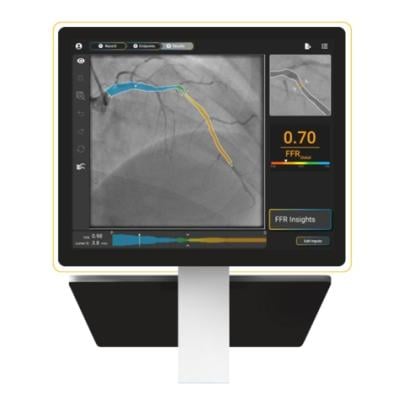
The X1 software is available as an add-on application to the HyperVue Imaging System. (Photo: SpectraWAVE)
Oct. 21, 2025 — SpectraWAVE, Inc., a medical imaging company focused on improving the treatment and outcomes for patients with coronary artery disease (CAD), recently announced U.S. Food and Drug Administration (FDA) 510(k) clearance for its wire-free, drug-free, single angiogram derived physiology product, X1-FFR. X1-FFR provides physicians physiology results from a single angiogram acquired via a direct real-time angiography feed, eliminating the need for network-based DICOM file management and transfer delays.
X1 features AI-enabled workflows, including automated vessel segmentation and frame suggestion, designed to simplify and speed up FFR results. X1 will be available as a software add-on to the HyperVue Imaging System, establishing a platform that combines FDA cleared angiogram-derived physiology with intravascular imaging.
"Physiology is essential for PCI outcomes, and replacing invasive pressure wires with non-invasive angiogram-derived physiology is better and safer for our patients," said Michael C. Kim, MD, Director of the Cardiac Cath Labs at Lenox Hill Hospital and Director of Interventional Cardiology in the Western Region of Northwell Health in NYC. "We've seen the advancement of angio-derived FFR over the past few years in clinical practice, but workflow and user variability have been a challenge with current technologies requiring multiple angiograms. SpectraWAVE’s X1-FFR technology represents the next stage in angio-derived physiology requiring only one view per vessel to derive an accurate FFR value. Our experience in clinical study cases has achieved many results in less than one minute. We are big believers in this technology, and we predict widespread usage not only within our large health system but nationally and internationally with the goal of identifying appropriate lesions for revascularization."
“HyperVue is already valued as a comprehensive, easy-to-use intravascular imaging product in the market. HyperVue with X1 becomes a one-of-a-kind central hub for the cath-lab offering both angiogram-derived physiology and imaging within the same platform,” said Eman Namati, PhD, Chief Executive Officer of SpectraWAVE. “By focusing on one vessel at a time, X1 can achieve accurate results from a single angiogram, while layering in state-of-the-art AI augmentation unlocks a number of key workflow benefits for customers, resulting in rapid and simple procedures. The clinical experience to date has been fantastic, and we’re excited to begin providing this to our customers and their patients. This marks our fifth FDA clearance in just over two years, reflecting our continued commitment to technological advancement to support patient outcomes in the cath lab.”
Approximately 2.5 million diagnostic angiograms and 1 million percutaneous coronary interventions (PCI) are performed annually in United States cardiac catheterization labs. Physiology, traditionally performed using pressure wires with and without adenosine, has become valuable to support treatment decision making during diagnostic angiograms, while intravascular imaging is used for the optimization of the treatment itself during PCI. HyperVue with X1-FFR is the first combination of FDA cleared products to provide a wire-free, drug-free angiogram derived physiology solution with an intravascular imaging product on the same platform to serve patients in the cath lab.
For more information, go to www.spectrawave.com


 January 15, 2026
January 15, 2026 









