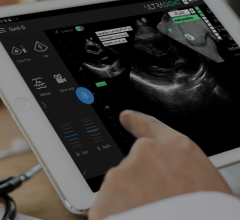
January 23, 2013 — St. Jude Medical Inc. announced European CE mark approval of its ViewFlex Xtra Intracardiac Echocardiography (ICE) Catheter. Designed for control and maneuverability during complex cardiac ablation procedures, the technology will be on display at the eighteenth annual Boston AF Symposium.
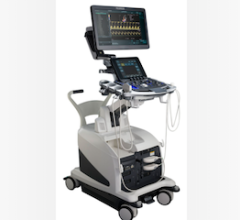
Offering a four-way directional tip, the ViewFlex Xtra ICE Catheter also has the added benefit of auto-lock steering, which allows for single-handed operation of the catheter to minimize repositioning. Paired with the ViewMate Z Intracardiac Ultrasound Console, the integrated products help clinicians better visualize, in real-time, a patient’s cardiac anatomy, including internal structure of the heart and blood flow direction. The high-resolution images and vivid color displays provide improved guidance for physicians.
“This technology offers crisp, clear visualization of the patient’s anatomy to help guide us in performing a variety of procedures such as radiofrequency ablation to treat irregular heart rhythms,” said Dhanunjaya Lakkireddy, M.D., cardiologist and professor of internal medicine at the University of Kansas Hospital in Kansas City, Kan. “The ViewFlex ICE Catheter’s easy to use, one-handed operation allows for a more efficient procedure.”
The catheter is inserted in the body through a small incision in the femoral vein (located in the leg) and guided to the heart. Featuring an ergonomic handle, the ViewFlex Xtra ICE Catheter offers improved maneuverability to enhance procedural workflow.
“The combination of the ViewFlex Xtra ICE Catheter and ViewMate Z Intracardiac Ultrasound Console in an integrated EP lab not only yields exceptional images, but more importantly allows physicians to focus on the patient and less on catheter manipulation,” said Frank J. Callaghan, president of the Cardiovascular and Ablation Technologies Division at St. Jude Medical.
The ViewFlex Xtra ICE Catheter received 510(k) clearance from the U.S. Food and Drug Administration (FDA) in 2012.
For more information: sjm.com

 October 09, 2025
October 09, 2025