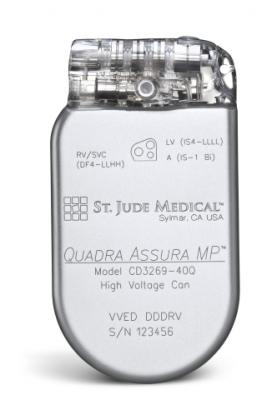
May 6, 2013 — St. Jude Medical announced its first enrollment in its MultiPoint Pacing clinical study to build upon its first- to-market quadripolar pacing system. Patients will be implanted with the Quadra Assura MP cardiac resynchronization therapy defibrillator (CRT-D) and Quartet lead to assess pacing in multiple locations in the heart.
The study will evaluate outcome benefits such as improved hemodynamics and cardiac function in heart failure patients who receive cardiac resynchronization therapy (CRT). MultiPoint pacing provides the ability to deliver two left ventricular (LV) pacing pulses, either simultaneously or sequentially, rather than the standard single pulse for each pacing cycle. This may be beneficial in further increasing the responder rates to CRT because it may capture a larger area of the cardiac anatomy by engaging areas around already damaged tissue.
“The MultiPoint Pacing trial is a study of patients who may not receive benefit or are unresponsive to standard CRT single-point pacing. We are evaluating whether MPP can increase the potential for a successful CRT outcome by pacing in multiple locations in the heart,” said Gery Tomassoni, M.D., director of electrophysiology at Baptist Health Lexington in Lexington, Ky.
The prospective, randomized, double-blind, multi-center clinical study will enroll more than 500 patients at 50 centers in the United States. Implanted patients will receive the single pacing pulse available with the existing quadripolar systems for the first three months. After three months, patients will be classified as being responders or non-responders to single-point CRT pacing and then will be randomized to either a single or multi-point pacing group. Patients will be monitored for an additional six months, at which time responder rates will be compared between patients receiving MultiPoint pacing and single point pacing.
For more information: www.sjm.com


 May 02, 2025
May 02, 2025 









