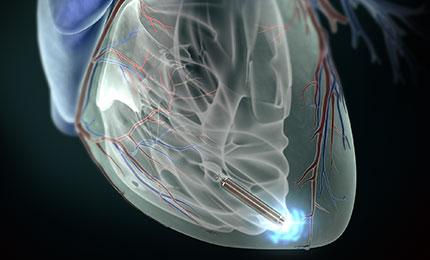
January 28, 2014 — Prof. Richard Schilling implanted St. Jude Medical Inc.’s Nanostim retrievable leadless
pacemaker at St. Bartholomew’s Hospital, London.
The Nanostim leadless pacemaker is designed to implant directly in the heart without visible surgical pocket, scar and insulated wires. Implanted via the femoral vein with a steerable
catheter, the device is designed to be fully retrievable to enable repositioning throughout implantation and after if necessary.
Maureen McCleave, a 77-year-old from Chingford who suffered from persistent
atrial fibrillation, became the first person in the U.K. to receive the Nanostim device. The procedure lasted eight minutes and McCleave is expected to recover fully.
The Nanostim leadless pacemaker is less than 10 percent the size of a conventional pacemaker. Lack of a surgical pocket and lead intends to improve patient comfort and can reduce complications, including device pocket-related infection and lead failure. The device is supported by the St. Jude Medical Merlin Programmer.
Initial results from the LEADLESS
study, a prospective, single-arm, multicenter study evaluating patients with the Nanostim leadless pacemaker, demonstrated overall device performance comparable to conventional pacemakers. Total implant procedure times averaged 28 minutes. The device battery is expected to have an average lifespan of more than nine years at 100 percent pacing, or more than 13 years at 50 percent pacing.
More than 4 million people worldwide have an implanted pacemaker or other cardiac rhythm management device, and an additional 700,000 patients receive the devices each year.
The Nanostim leadless pacemaker has CE marking and is available in select European markets, and is not for sale in the United States.
For more information: www.sjm.com