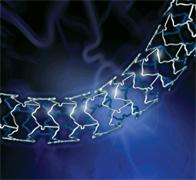
September 21, 2010 – A new drug-eluting stent system has received CE mark approval for diabetic patients and those experiencing a heart attack.
The PROMUS element everolimus-eluting stent system, made by Boston Scientific, can be used in patients with concomitant diabetes mellitus as well as those suffering from an acute myocardial infarction (AMI).
The stent features a platinum chromium alloy, which helps offer greater radial strength and flexibility while reducing stent recoil. The geometry helps create consistent lesion coverage and drug distribution while improving deliverability.
CE Mark approval for PROMUS occurred in October 2009 and for the TAXUS ElementTM paclitaxel-eluting stent system in May, which included a specific indication for the treatment of diabetic patients. Both systems incorporate the same platinum chromium alloy, stent design and catheter delivery system.
Boston Scientific expects U.S. Food and Drug Administration (FDA) approval for TAXUS in 2011 and for PROMUS in 2012.
For more information please visit: www.bostonscientific.com


 November 24, 2025
November 24, 2025 









