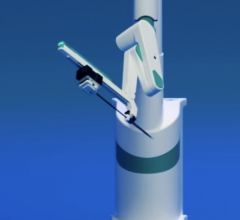
February 9, 2012 — Stereotaxis Inc. said its Vdrive robotic navigation system, which provides physicians the ability to remotely manipulate traditionally non-robotic catheters, is growing in popularity and is expected to surpass 500 clinical procedures in Europe in February. The company also announced it has received regulatory clearance from Health Canada to commercially market the device in the country.
Since the initial product release in Europe in 2011, the Vdrive system has been installed in nine centers, with units to be installed in additional centers during the first quarter of 2012. The initial nine have performed 473 clinical cases with approximately 80 percent being completed in the left atrium of the heart.
Numerous cases have been performed with the Vdrive system and clinical feedback continues to be very positive," said Michael Kaminski, president and CEO of Stereotaxis. "With the Health Canada market clearance for the Vdrive system, and our planned new Vdrive installations in Europe and Canada this year, we are well-positioned to drive the growth and further adoption of this exciting technology in electrophysiology labs in these important markets."
In a fully remote procedure environment, the Vdrive system increases the clinical techniques available to the physician and reduces the need to re-enter the sterile field to adjust devices. Initial clinical data from European physicians demonstrates that this simplification saves 30 minutes or more in robotic procedures, depending on individual clinical technique. Furthermore, the addition of robotic diagnostic catheter manipulation is another step to improve device control. Stereotaxis' broad Epoch solutions portfolio also includes precise magnetic control of ablation catheters with Niobe ES and integrated display and control of multiple lab technologies with the Odyssey clinical information management system.
The design of the Vdrive system allows the robotic hardware to adapt to different clinical techniques depending on the disposable adaptor that is attached to the arm:
- V-Loop circular catheter manipulator allows control of circular diagnostic catheters, primarily in left atrial procedures.
- V-CAS catheter advancement system allows advancement and retraction of the magnetic catheter as well as robotic manipulation of catheter introducer sheaths that are already in use during the procedure.
- V-CAS Deflect catheter advancement system is a more advanced device that includes an integrated robotic deflectable sheath.
Initial positive results from multiple physician users confirmed the significant clinical value delivered by the Vdrive system related to procedure efficiency.
For more information: www.stereotaxis.com


 January 27, 2026
January 27, 2026