August 22, 2014 — Sunshine Heart Inc. announced that the current condition of a heart failure patient implanted with the C-Pulse System improved after six weeks of treatment, allowing him to attend his daughter's wedding. The patient, Ross Swift, a 54 year-old grandfather from Devon, U.K., received C-Pulse at Harefield Hospital in London and is participating in the OPTIONS HF study, a post-market surveillance study being conducted in Germany and the United Kingdom. The C-Pulse system received the CE mark in 2012. The story was reported in an article published in The Daily Mail, titled, “Me and My Operation: Pump that squeezes your arteries to help fight heart failure.” The surgical procedure was carried out by Andre Simon, M.D., director of transplantation at Royal Brompton & Harefield NHS Foundation Trust.
"When I received the C-Pulse System, immediately I could tell there was a difference. When I breathed, it felt clearer. I had more color in my face and my family said my attitude changed," said Swift. "I feel more positive. I find day-to-day activities, which used to wear me out, much easier to get on with and recover from afterwards.
"The wedding was amazing. Everybody was crying knowing what we had been through. Walking my daughter down the aisle was something a few months ago we didn't believe was possible."
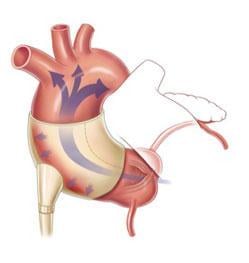
The C-Pulse System's design is based on an extra-aortic balloon cuff counter-pulsation therapy that is applied outside of the bloodstream and assists the heart by reducing the workload of the left ventricle. During inflation of the balloon cuff, blood flow is increased to the coronary arteries, thereby providing additional oxygen, which is vital to a failing heart. During deflation of the balloon cuff, the workload or pumping required by the left ventricle is reduced. The balloon's inflation and deflation is synchronized to the patient's heart rhythm, similar to a pacemaker.
"The C-Pulse System is a treatment option that has not previously been available for this particular group of heart failure patients," said Simon. "The aim of implanting the system is to help slow the deterioration of a failing heart, which can relieve heart failure symptoms and improve their quality of life. At Harefield Hospital we see lots of patients with heart failure who are limited in their everyday activities, such as walking to the shops or taking part in family activities, but many of these patients are not sick enough to need a heart transplant."
Sunshine Heart is currently enrolling patients in clinical studies in the United States and Europe for treatment of moderate to severe heart failure. The OPTIONS HF clinical study is a post-market, multi-center, prospective, surveillance study in up to 15 European centers. The study is designed to observe long-term clinical outcomes of heart failure patients treated with the C-Pulse System. Concurrently, the company's COUNTER HF U.S. pivotal study is underway and designed to achieve commercial approval of the C-Pulse System in the United States.
The C-Pulse Heart Assist System, or C-Pulse System, an investigational device in the United States, Canada and countries that do not recognize the CE mark approval, utilizes the scientific principles of intra-aortic balloon counterpulsation applied in an extra-aortic approach to assist the left ventricle by reducing the workload required to pump blood throughout the body, while increasing blood flow to the coronary arteries. Combined, these potential benefits may help sustain the patient's current condition or, in some cases, reverse the heart failure process, thereby potentially preventing the need for later-stage heart failure devices, such as left ventricular assist devices (LVADs), artificial hearts or transplants. It may also provide relief from the symptoms of Class III and ambulatory Class IV heart failure and improve quality of life and cardiac function. Based on the results from our feasibility study, we also believe that some patients treated with our C-Pulse System will be able to be weaned from using the device due to sustained improvement in their heart failure condition as a result of the therapy.
For more information: hfclinicalstudy.com/index.htm, clinicaltrials.gov/ct2/show/NCT01872949?term=NCT01872949&rank=1


 February 03, 2026
February 03, 2026 









