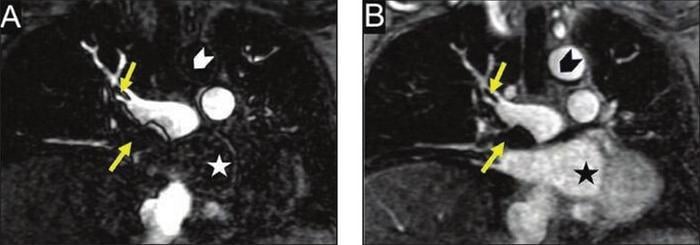
Patient was found to have deep vein thrombosis. Gadolinium-enhanced MRA was performed to assess for pulmonary embolus (PE). Coronal contrast-enhanced T1-weighted images in pulmonary arterial phase (A) and low flip-angle delayed phase (B) show PE (arrows, A and B). Delayed-phase image also shows cardiac chamber (star) and ascending thoracic aorta (chevron). Multiphase acquisition of MRA during inflow and outflow of contrast agent from pulmonary arteries is in distinction to single acquisition of CTA and may facilitate obtaining optimal enhancement of pulmonary arteries. Image courtesy of AJR
July 3, 2023 — According to an accepted manuscript published in ARRS’ own American Journal of Roentgenology (AJR), preferential use of pulmonary MR angiography (MRA) for diagnosing pulmonary embolus (PE) in the general population helped conserve iodinated contrast media during the 2022 shortage.
“This single-center experience demonstrates use of pulmonary MRA as a practical substitute for pulmonary CTA in emergency settings,” concluded lead investigator Jitka Starekova, MD, from the radiology department of the University of Wisconsin in Madison.
Starekova et al.’s study included all CTA and MRA examinations performed to exclude PE at their large academic medical center (University of Wisconsin-Madison Hospital and Clinics) from April 1 through July 31 (18 weekly periods) in:
2019 (before the COVID-19 pandemic and contrast media shortage),
2021 (during the pandemic, though before the shortage),
2022 (during both the pandemic, as well as the shortage).
To help preserve iodinated contrast media, from early May through mid-July of 2022, MRA served as the preferred test for PE diagnosis. Upon reviewing CTA and MRA reports, Starekova and her AJR colleagues estimated iodinated contrast media savings via preferential MRA usage.
Ultimately, preferred use of pulmonary MRA to diagnosis PE among the general population conserved (from April 1 to July 31, 2022) an estimated 27 liters of iohexol (Omnipaque) 350mg/ml. During weeks 8-11, more MRA examinations (range, 45-63) than CTA examinations (range, 27-46) were performed.
Noting their AJR accepted manuscript evaluated MRA for PE diagnosis in “an all-comer real-world setting,” the present study also includes a larger sample size in comparison to previous research.
For more information: www.arrs.org

 March 20, 2024
March 20, 2024