December 23, 2014 — Tendyne Holdings Inc. announced that in November 2014, the Tendyne Transcatheter Mitral Valve Implantation system (TMVI) was successfully implanted in the first patient of the Tendyne Feasibility Study. This was the first patient enrolled in a three continent, multicenter trial, which aims to generate insight into the safety and performance of the Tendyne device in inoperable patients suffering from mitral regurgitation.
David Muller, M.D., and Paul Jansz, M.D., performed the implantation at St. Vincent’s Hospital in Sydney, Australia. Commenting on the procedure, Muller said, “The device was implanted transapically without cardiopulmonary bypass and performed as intended by completely eliminating mitral regurgitation. The patient recovered quickly and was released from the hospital on day five. We are now monitoring the patient as part of the Tendyne Feasibility Study. We believe the device has the potential to offer a very effective solution for patients at high risk for conventional mitral valve surgery”.
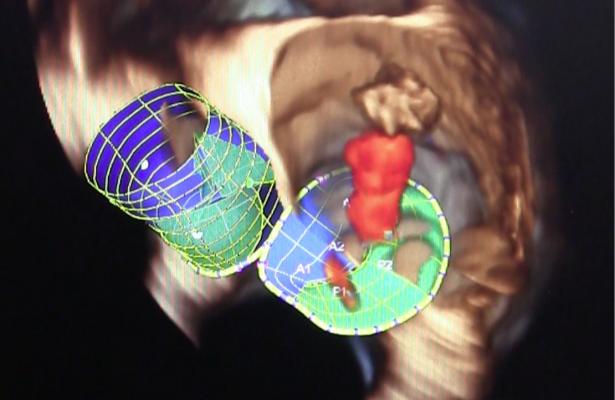
The Tendyne TMVI is a fully retrievable and repositionable, apically tethered tri-leaflet porcine pericardial valve sewn onto a nitinol frame that was specifically designed to address the complex mitral anatomy of functional, degenerative and mixed etiology mitral regurgitation. Left untreated, mitral regurgitation can lead to heart failure and death.
For more information: www.tendyne.com


 December 24, 2025
December 24, 2025 









