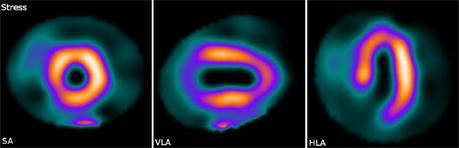
Dual-isotope imaging: reduced-dose, shorter-scan-time stress images acquired with 20 mCi Tc-99m at 10 sec/frame. Photo courtesy of Mayo Clinic.
March 9, 2010 – As an alternative to the technetium (Tc-99M) isotope used in many nuclear imaging studies, UltraSPECT is advocating the use of thallium isotope (TI-201) imaging as an alternative. Due to the impact of the Canadian reactor shutdown combined with the recently announced six-month repair plan of the Dutch reactor, the global shortage of Tc-99m is expected to remain grave in the next few months, leaving nuclear medicine in a precarious position. The shortage means there is an inability to provide patients with needed tests, longer delays in exam scheduling, use of reduced-dose imaging protocols, and loss of business to other imaging modalities such as CTA, echo and cardiac PET. The company offers two cardiac products, Xpress.Cardiac and Xpert3.Cardiac, which support Tl-201 imaging. These products utilize UltraSPECT's proprietary WBR image reconstruction capabilities to enable reduced-dose and enhanced-resolution thallium imaging. The products allow the utilization of lower dose imaging protocols using Tc-99m with no compromise in terms of imaging acquisition times and diagnostic value. They also support reduced-dose and enhanced-resolution Tl-201 imaging, helping alleviating the impact of the technetium shortage while reducing patient radiation exposure. UltraSPECT products feature connectivity to the imaging systems of all three major nuclear medicine camera manufacturers. “UltraSPECT has allowed us to acquire high-quality gated SPECT images with Tl-201, without increasing scan times,” said John Zeitz, M.D., chief of nuclear medicine at Beaumont Hospital in Troy, Mich. “Previously, we would have to increase our imaging time by 50 percent in order to achieve decent image quality for the gated cine reconstruction." The one-day dual-isotope (Tl-201 and Tc-99m) protocol performed with the Xpress3.Cardiac combines reduced doses with shorter scan times. Reduced-dose, shorter-scan-time rest Tl-201 imaging is followed by reduced-dose, shorter-scan-time Tc-99m stress imaging, rendering the procedure safer and more comfortable for the patient, while ensuring sustained/increased patient throughput for the practice: • Rest: Tl-201, 2 mCi (female) / 3 mCi (male); 20sec. per stop. • Stress: Tc-99m, 20 mCi (female) / 30 mCi (male); 10sec. per stop. UltraSPECT’s Xpress.Cardiac, Xpress3.Cardiac, and Xpress/Xact.BoneTM products are distributed in the USA exclusively by Cardinal Health. UltraSPECT products are distributed in the USA by Cardinal Health (www.cardinalhealth.com). For more information: www.ultraspect.com


 March 25, 2025
March 25, 2025 









