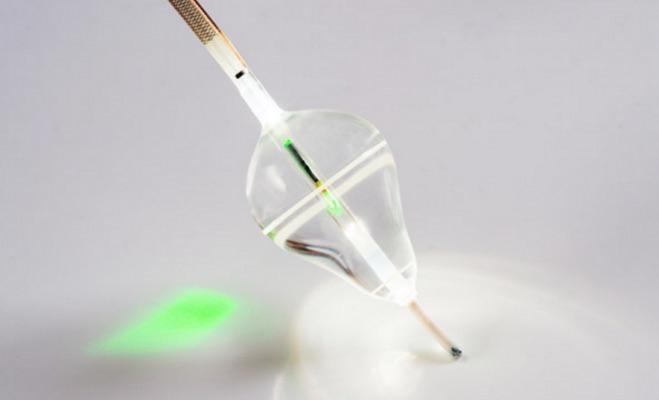
August 7, 2017 — CardioFocus Inc. announced that its HeartLight Endoscopic Ablation System is being featured in three new major clinical studies currently underway. Collectively, up to 1,000 AF patients will be enrolled in these studies.
The first study, titled, "CardioFocus vs. Contact Force Guided Pulmonary Vein Isolation in Paroxysmal Atrial Fibrillation (CF²)," is a prospective, multi-center, randomized study comparing the acute procedure and safety outcomes as well as long-term clinical outcomes of two groups of patients. One group is being treated with the HeartLight System and the other with a commercially available contact force sensing irrigated radiofrequency (RF) ablation catheter plus, at the operator's discretion, 3-D electroanatomical mapping (EAM) for the treatment of paroxysmal atrial fibrillation (AF). The study will recruit up to 350 patients at approximately 10 centers throughout Europe. Andreas Metzner, M.D., from Asklepios Klinik St. Georg in Hamburg, Germany is leading the study.
A second randomized study, led by the Cardiovascular Center Bethanien (CCB) in Frankfurt am Main, Germany, will enroll a total of 400 patients in Europe. 100 patients with paroxysmal AF will be treated with the HeartLight System and 100 paroxysmal AF patients will be treated with the Cryoballoon. Additionally, 100 persistent AF patients will be treated with the HeartLight System and 100 persistent AF patients will be treated with the Cryoballoon. Comparisons across these groups will be analyzed. Boris Schmidt, M.D., and Julian Chun, M.D., from CCB are the principal investigators for this study. Both previously authored a paper comparing the performance of the HeartLight System to ablation with the Cryoballoon in the paroxysmal AF population. That study showed a 73 percent success rate (freedom from AF) at 12 months with the HeartLight System as compared with 63 percent with the Cryoballoon.
The third study, titled, "The Post-Approval Study (PAS) of the HeartLight Endoscopic Ablation System for the Treatment of Atrial Fibrillation," is evaluating the clinical outcomes of a cohort of patients treated during commercial use of the HeartLight System to confirm results of the successful U.S. clinical pivotal study and study additional research questions. The study will include up to 250 enrolled patients and up to 25 sites in the United States.
Results from these three studies will provide additional clinical evidence on the performance of the HeartLight System in the AF population, helping to support its usage as a gold-standard catheter ablation therapy, according to CardioFocus.
These studies follow positive data presented at the Heart Rhythm Society's (HRS) 38th Annual Scientific Sessions, May 10-13 in Chicago. Outcomes data on the HeartLight system were presented by Pieter Koopman, M.D., of the Heart Center Hasselt in Hasselt, Belgium, Scott Gall, M.D., of the Lancashire Cardiac Centre in the United Kingdom, Schmidt from CCB, Aditi Naniwadekar, M.D., of the Helmsley Center for Cardiac Electrophysiology at Mt. Sinai Hospital, New York, and Rosa M. Figueras i Ventura, M.D., of the Hospital Clinic of Barcelona.
"The data continue to reinforce the chronic efficacy of laser balloon ablation with the HeartLight System in our most recent single-center comparative analysis showing freedom from AF at one year of 93 percent," said Koopman.
The HeartLight System's direct visualization, titratable laser energy and universal balloon design offer a new treatment for pulmonary vein isolation (PVI) procedures. The device received CE Mark in 2009 and U.S. Food and Drug Administration (FDA) approval in April 2016. In the U.S., the HeartLight System is indicated for the treatment of drug refractory recurrent symptomatic paroxysmal AF.
Watch the VIDEO ”Current State of Atrial Fibrillation Ablation Technologies,” and interview with Hugh Calkins, M.D., FACC, FAHA, FHRS, director of cardiac arrhythmia services and professor of medicine at Johns Hopkins Hospital.
For more information: www.cardiofocus.com


 February 06, 2026
February 06, 2026 









