February 6, 2014 — Transcatheter Technologies GmbH, a medical device company, is developing a third-generation transcatheter aortic
valve implantation (TAVI) system, Trinity. An independent laboratory completed advanced wear testing (AWT) of the company’s Trinity valve prosthesis. AWT of the Trinity heart valve has completed 600 million cycles, or an estimated 15 years of durability testing.
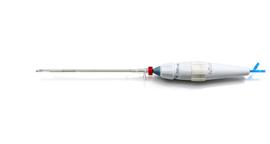
The Trinity aortic valve is designed to be positioned precisely or repositioned implantation.
“In our study, Trinity’s sealing cuff continues to provide outstanding follow-up results without PVL (paravalvular leak), a frequent complication of TAVI,” said Prof. Dr. Christian Hengstenberg, a cardiologist at the German Heart Center, Munich, Germany. “Equally important, the Trinity aortic valve is designed to reduce the risk of atrio-ventricular (AV) block significantly through supra-annular positioning of the Trinity valve.”
The Trinity aortic valve prosthesis is comprised of a bovine pericardium valve with porcine pericardium-sealing cuff that is mounted on a self-expanding Nitinol frame. The valve is pre-mounted on a detachable catheter tip. Trinity’s features enable controlled positioning and true repositioning without foreshortening. The valve prosthesis is protected during folding of the stent. This Zero Pressure Crimping is expected to improve valve durability and broaden the application of transcatheter valve implantations across a larger patient population.
Trinity is not approved for use in the United States.
For more information: transcathetertechnologies.com