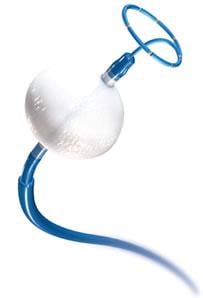
Image courtesy of Medtronic
January 12, 2015 — Medtronic Inc. announced the initiation of a randomized clinical trial in Europe to assess the benefits of ablation using the Medtronic Arctic Front Advance cryoballoon as a first-line treatment for atrial fibrillation (AF) patients. While medication is currently considered first-line treatment for AF, clinical research indicates that half of all patients with symptomatic disease do not respond to drug therapy; earlier ablation may improve treatment effectiveness.
The Cryo-FIRST (Catheter Cryoablation Versus Antiarrhythmic Drug as First-Line Therapy of Paroxysmal Atrial Fibrillation) trial will evaluate the procedural success and clinical outcomes of patients with paroxysmal AF (PAF) undergoing cryoballoon ablation as an initial treatment compared with medication. It will include approximately 218 patients in up to 12 centers across Europe. Enrollments have occurred at Heart Rhythm Management Centre, UZ Brussel-VUB Brussel (Brussels, Belgium); Clinique Saint Gatien (Tours, France); CHU d' Amiens (Amien, France) and Kerckhoff-Klinik (Bad Nauheim, Germany).
The most recent European guidelines highlight that ablation should be considered as first-line therapy in selected patients with symptomatic PAF. Cryoballoon ablation is not approved as a first-line treatment in the United States.
Cryoablation involves a minimally invasive procedure that creates lesions by freezing tissue in the heart's upper chambers, traditionally around the pulmonary veins, to block the electrical signals that trigger erratic heart rhythms. Pulmonary vein isolation (PVI) is a standard treatment for patients in the early stages of AF when the triggers come largely from these veins. However, as the disease progresses, it becomes more complicated to treat, with lower long-term success rates.
For more information: www.medtronic.com


 January 05, 2026
January 05, 2026 









