April 22, 2011 – The American Diabetes Association (ADA) has invited NewCardio to present the results of a key study of the company’s urgent care solution, my3KG. Results showed that the solution had substantially greater accuracy than expert cardiologists' interpretation of standard 12-lead ECG (12L ECG) in diagnosing acute myocardial infarction (AMI) in diabetic patients.
The presentation will be made at the 71st Annual ADA Scientific Sessions to be held in San Diego, Calif., June 24-28, 2011. In addition, the ADA has selected this presentation to be showcased in the Guided Audio Poster Tour, which features expert moderators that share their perspectives with attendees, highlighting novel and important developments in the field.
"We are delighted to present our study results at this important gathering of diabetes experts, and particularly pleased that the ADA program committee chose to feature our presentation in a moderated poster session,” said Ihor Gussak, M.D., Ph.D., NewCardio's chief medical officer. “Accurate and timely AMI diagnosis is a matter of great concern for diabetologists, and we believe our results show that my3KG can play a major role in solving it. The moderated poster session provides the opportunity to present our results directly to leading diabetes experts, and have one-on-one interactions that will allow us to discuss and explain the importance of our results in detail."
Diabetics with AMI represent a very large and growing patient group, and currently affect more than 10 percent of adults in the United States. Moreover, the incidence of AMI is two to four times greater in diabetics than in the general population, according to ADA and American Heart Association statistics. Accurate and timely diagnosis of AMI is notoriously difficult in diabetics, largely because the standard 12L ECG is often inaccurate, inconclusive or non-diagnostic in this patient group. To address this problem, investigators at the University of Kansas Medical Center, in collaboration with NewCardio physician-scientists, obtained detailed clinical and 12L ECG data on 155 consecutive diabetic patients with suspected AMI, and evaluated the diagnostic performance of my3KG in this patient group.
The study showed that my3KG had 42 percent greater sensitivity than expert cardiologist interpretation of 12L ECG for early detection of AMI, with equal or better specificity. Based on these important results, NewCardio believes use of my3KG in this patient group may facilitate more accurate and timely diagnosis of AMI in diabetic patients, thereby improving clinical outcomes.
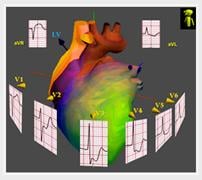
The 3-D ECG platform technology dramatically improves the accuracy and significantly increases the diagnostic value of the standard 12L ECG. NewCardio is developing the my3KG solution for urgent diagnosis of serious cardiac conditions, including AMI. In clinical studies to date, the my3KG has shown substantially improved diagnostic accuracy relative to the standard 12L ECG, particularly in the most diagnostically challenging patients.
The my3KG is not currently marketed for sale in the United States. The company intends to file an application for 510(k) approval with the U.S. Food and Drug Administration (FDA) in 2011/2012.
For more information: www.newcardio.com


 January 15, 2026
January 15, 2026 









