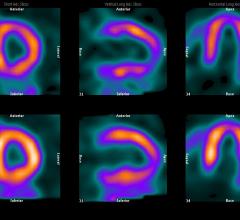
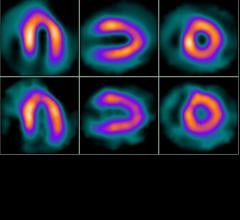
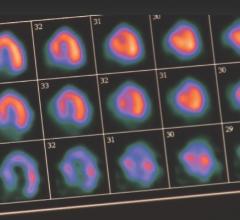
February 24, 2012 — Positron Corp., through its wholly owned subsidiary, Manhattan Isotope Technology LLC (MIT), announced it had received its first shipment of strontium-82 (Sr-82) from the ARRONAX Cyclotron Facility in Nantes, France.
This historic event marks the first shipment of Sr-82 to be received by a private, non-government entity in the United States in support of Sr-82 production. The receipt of this material also marks the beginning of MIT's validation efforts in order to qualify Sr-82 product material as active pharmaceutical ingredient (API).
Over the next six months MIT will continue to obtain Sr-82 target solutions from foreign irradiators at Positron’s Strontium processing facility in Lubbock, Texas. MIT will conduct the critical processing procedures for final purification into API grade Sr-82. Validation of the API grade Sr-82 product material is key to the Positron/MIT 2012 Drug Master File submission to the United States Food and Drug Administration (FDA).
"With the receipt of Sr-82 from ARRONAX, Positron and MIT have achieved an incredible milestone," states MIT president, Jason Kitten. "Up until this point in time, the Department of Energy was the only organization in the United States capable of processing Sr-82 material. By executing our plans, MIT is well positioned to meet the Drug Master File qualifications, which will further advance and accelerate Positron's Sr-82 mission. Positron continues its progress and is on schedule to meet 2012 Sr-82 supply objectives. Positron's goal of solidifying and advancing cardiac PET's progression in the U.S. is well underway."
For more information: www.positron.com


 March 25, 2025
March 25, 2025