March 6, 2014 — The Valley Hospital in Ridgewood, N.J., is among a few hospitals in the nation to implant a tiny wireless heart monitor expected to have impacts for patients and doctors.
Indicated for use as a diagnostic tool for people suffering from unexplained fainting, dizziness, palpitations or shortness of breath, the device can also help doctors determine if a patient has atrial fibrillation, the most common form of heart rhythm abnormality.
Nick Rotonda of Upper Saddle River, N.J., was the first patient to receive the device at Valley and is counting on it to monitor whether he has any signs of atrial fibrillation, which could increase his risk for a stroke.
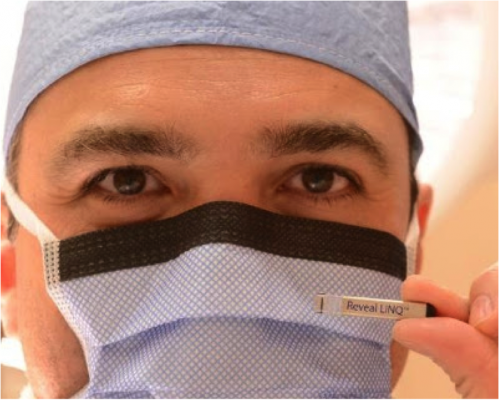
About one-third the size of a AAA battery and almost 90 percent smaller than similar devices, the Reveal Linq implantable cardiac monitor is slipped just beneath the skin with a syringe-like device through an incision less than half an inch long. It continuously and wirelessly monitors the heart for up to three years and notifies physicians if patients have significant cardiac events. It is also MRI-compatible.
"It takes about 5 minutes to implant the device using a local anesthetic," said cardiac electrophysiologist Dan L. Musat, M.D., attending physician at Valley's Arrhythmia Institute, part of the Valley Heart and Vascular Institute. "There is no need for general anesthetic, the device is not visible in most patients and patients go home after about an hour," said Musat, who performed Valley's first procedure.
The device has the ability to communicate wirelessly via a small tabletop remote monitoring station while patients sleep, allowing them to continue living their lives normally, even away from home.
"This is one of the most innovative technologies to emerge in cardiology in the last decade," said Suneet Mittal, M.D., director of the electrophysiology lab at Valley. "It is so discreet that most patients will not even know it is there and can go about their lives without interruption or discomfort. It truly is a game changer."
"This monitor gives me peace of mind because I know that if I have an episode it will alert my doctor so he can address it," Rotonda said.
Medtronic's Reveal Linq was approved by the U.S. Food and Drug Administration in February.
For more information: www.medtronic.com


 July 22, 2025
July 22, 2025