February 9, 2016 — Valtech Cardio Ltd. announced that it has received German Neue Untersuchungs und Behandlungsmethoden (NUB) Status 1 approval for the Cardioband Mitral Reconstruction System, its flagship device for addressing mitral regurgitation in heart failure patients. NUB approval for the procedure grants reimbursement for Cardioband therapy in 70 leading hospitals in Germany.
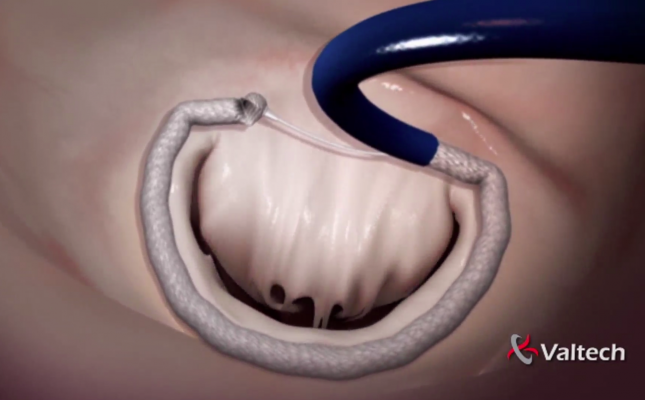
The Cardioband System combines a reconstruction implant, similar to those used in traditional surgical annuloplasty devices, with a transfemoral, transseptal delivery system. Sutureless connection of the implant to the mitral annulus is achieved using specially designed anchors. Reshaping of the mitral annulus to eliminate mitral regurgitation (MR) is done under physiological conditions and echocardiographic guidance for optimal results.
Cardioband received CE Mark approval after clinical trial results demonstrated the device is a safe and efficacious intervention option for patients with functional mitral regurgitation (FMR).
For more information: www.valtechcardio.com


 December 24, 2025
December 24, 2025 









