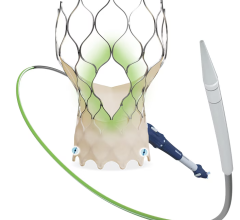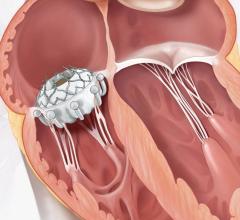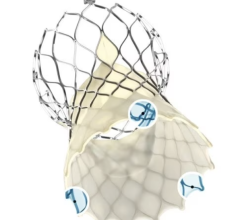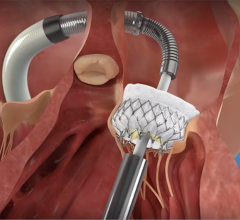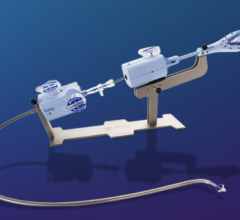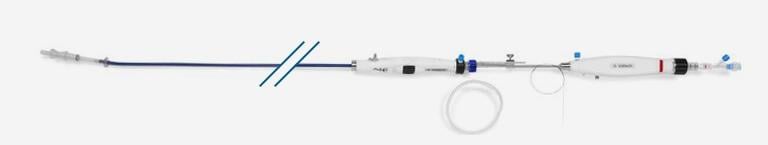
September 14, 2015 — Startup cardiovascular device manufacturer Valtech Cardio Ltd. announced it has received CE Marking for its Cardioband mitral reconstruction system (Cardioband), a proprietary, implantable mitral reconstruction device with a transfemoral, transseptal delivery system for mitral valve repair.
The designation, received from Dekra, Valtech's notified body in the European Union, was based on the results of a multicenter feasibility trial that demonstrated the safety and effectiveness of Cardioband in mitral valve repair and will allow Valtech to market and sell the device in the European Union. Results of the study demonstrated that Cardioband is a highly effective first-line treatment option for reducing mitral regurgitation (MR) and improving quality of life scores.
Valtech will officially unveil the Cardioband at the upcoming PCR London Valves meeting, Sept. 20-22, in Berlin.
MR is a condition in which the mitral valve leaflets fail to close properly, allowing backflow of blood from the left ventricle into the left atrium during systole. Left untreated, severe MR can eventually lead to a meaningful deterioration in cardiac function and, eventually, death. Approximately 4.2 million patients in the United States alone are affected by mitral valve disease, which represents a several-billion-dollar global market opportunity.
The Cardioband mitral reconstruction system enables surgical-like repair of the mitral valve annulus via a transfemoral, transseptal delivery system, allowing for real-time adjustment on a beating heart. The transcatheter, supra-annular approach does not interfere with the mitral valve leaflets or chordae, and does not preclude subsequent treatment options if they become necessary.
In a multicenter feasibility trial including more than 50 patients, Cardioband was shown to significantly reduce annular size, with significant improvement in MR. After six months of follow-up, 82 percent of patients (n = 22) were categorized in New York Heart Association (NYHA) Class I-II, with significant improvement of quality of life (Minnesota Living With Heart Failure Questionnaire) score of 38 to 18 [p<0.05]; and had six-minute walk test score of 250 to 322 [p<0.05]). At 12 months' follow-up, 94 percent of patients (n = 17) had sustained MR less than or equal to 2+.
"The future of functional mitral regurgitation treatment lies in the repair-and-replace paradigm," commented Francesco Maisano, M.D., first user of the Cardioband procedure and chairman and professor of cardiovascular surgery at The University Hospital of Zurich. "MR repair with Cardioband can be the first-line therapy for severe MR patients. Additionally, early-stage repair can support ventricular reverse remodeling while keeping the options open for the patient, supporting the technology's use at earlier stages."
The Cardioband System combines a reconstruction implant, similar to the surgical annuloplasty devices, with a transfemoral, transseptal delivery system. Connection of the implant to the mitral annulus is sutureless, using specially designed anchors. Reshaping of the mitral annulus to eliminate MR is done under physiological conditions and echocardiographic guidance for optimal results.
For more information: www.valtechcardio.com


 July 08, 2024
July 08, 2024