August 24, 2009 – Prescient Medical Inc. said today it received European CE mark clearance to commercialize its vProtect Luminal Shield Stent System.
The device is now approved for use in improving coronary artery luminal diameter in patients with symptomatic ischemic heart disease.
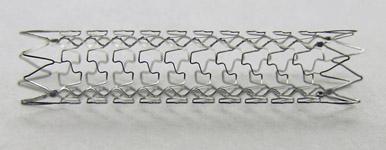
The novel vProtect Luminal Shield has the potential to change the way interventional cardiologists treat arterial plaques. The vProtect Luminal Shield is designed to minimize arterial injury and its consequences, allowing the vessel to regain and maintain healthy physiological function. The device is a low radial force, thin-strut, self-expanding nitinol mesh, or "Shield" that is designed specifically for softer plaques.
The company developed the stent as a prophylactic treatment for vulnerable plaques that are identified in the coronary arteries.
"We are very pleased with the performance of the Shield in our clinical study program," said principal investigator Juan F. Granada, M.D., Medical Director, Skirball Center for Cardiovascular Research, Cardiovascular Research Foundation, New York, NY. "We achieved excellent technical results. In this clinical series there were no periprocedural MACE events and we achieved device delivery success in all the cases. The Shield continues to demonstrate a very favorable efficacy profile in all cases evaluated to date."
The company is the recipient of the 2009 Frost and Sullivan Award for Product Innovation of the Year Award for vProtect Luminal Shield.
For more information: www.prescientmedical.com


 November 24, 2025
November 24, 2025 









