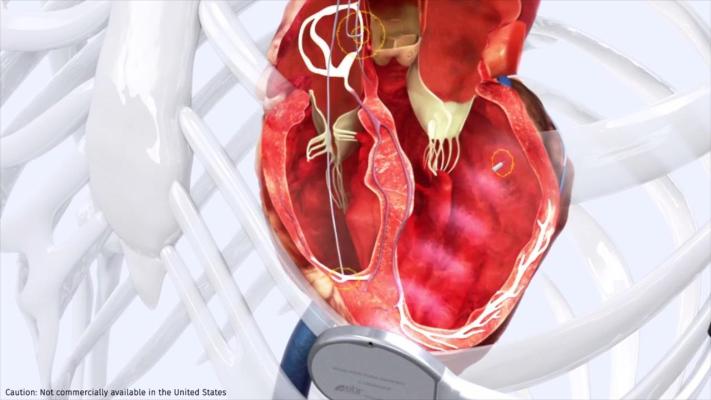
May 22, 2023 — Results from a pivotal clinical trial found a leadless pacemaker can deliver cardiac resynchronization therapy (CRT) among patients who were not able to be treated with conventional CRT and epicardial leads. The novel WiSE CRT system removes the possibility of lead complications and provides physicians with more freedom to target the pacing site within the endocardium of the heart’s left ventricle when implanting the device. The results from this late breaking clinical trial were presented today during Heart Rhythm 2023.
Heart failure is a progressive disease that occurs when the heart can’t pump enough blood to meet the body’s demands. For many patients with heart failure, their two ventricles end up beating at different times in a dyssynchronous rhythm. The current standard of care to help achieve synchrony is CRT, which uses pacing to synchronize the contraction of the right and left ventricles. However, lead-based pacemakers have known limitations and up to 30% of patients fail to benefit from this therapy.1
The SOLVE-CRT study is a prospective, international, multicenter pivotal trial that included a randomized part and the subsequent single-arm part. The randomized part of the study was initially intended to enroll 350 patient participants for three indications: patients who were non-responders (NR), those previously untreated, including acute and chronic lead failures (PU), and patients undergoing high-risk upgrades (HRU) from existing implanted cardioverter defibrillators (ICD). All participants underwent device implantation and were then randomized in 1:1 ratio to Treatment (system turned ON) or Control (system turned OFF) groups. A total of 108 participants were enrolled by March 2020 when enrollment was halted due the COVID-19 pandemic.
The single arm part of the study was approved by the US FDA during the COVID-19 pandemic and was designed to enroll up to an additional 192 participants within two indications: PU and HRU. An interim analysis was conducted after 75 participants were enrolled. Data from all participants in both parts were included in the interim safety analysis (n=183). The primary safety endpoint for the SOLVE-CRT study was freedom from Type I (device & procedure-related) complications through 6 months. Efficacy analysis included all participants in the single arm (n=75) and those randomized to treatment from the PU and HRU groups only (n=25), for a total of n=100. The primary efficacy endpoint was the improvement in Left Ventricular Systolic Volume (LVESV).
WiSE CRT was shown to be safe with an 80.9% freedom from Type I complications (p < 0.001). In addition, there was a significant improvement of 16.4% in Left Ventricular End Systolic Volume (p = 0.003). Since both primary endpoints were met at this interim analysis, study enrollment will be concluded early.
"Because heart failure is a progressive disease, patients who fail conventional CRT experience worsening symptoms that can result in repeated hospitalizations and reduced life expectancy. The results of SOLVE-CRT offer a new hope for patients with hard-to-treat heart failure," said Jagmeet Singh, MD, DPhil, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, and Co-PI of the study along with Dr. Mary Norine Walsh, MD, Ascension St. Vincent Heart Center, Indianapolis, Indiana. "Endocardial leadless pacing will allow physicians to choose where to implant the device within the left ventricle and the ability to tailor the therapy directly to each individual patient’s needs."
The sponsor of this trial, EBR Systems, Inc., is currently working to submit a Premarket Approval (PMA) to the FDA for U.S. approval.
For more information: www.hrsonline.org
Find more HRS23 conference coverage here
Reference:
1 "Cardiac Resynchronization Therapy." Cardiac Resynchronization Therapy | Johns Hopkins Medicine, 19 Nov. 2019, www.hopkinsmedicine.org/health/treatment-tests-and-therapies/cardiac-resynchronization-therapy.
Related Leadless CRT Content:
Leadless CRT Can Deliver Left Bundle Branch Area Pacing for Cardiac Resynchronization
VIDEO: How to Implant a Leadless CRT LV Pacing System
Leadless Endocardial CRT Pacing Effective for Heart Failure Patients
What is New in Electrophysiology Technologies
Leadless Pacemaker Stimulates Heart With Wireless Transmission of Energy


 July 30, 2024
July 30, 2024