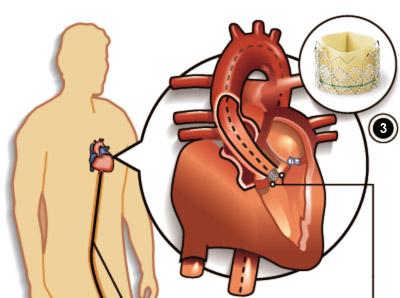
November 6, 2009 – Edwards Lifesciences Corp. today announced the first successful Japanese implants of a transcatheter aortic heart valve using its Edwards SAPIEN valve.
The transfemoral and transapical valve implantations were performed by Osaka University last month in preparation for Osaka’s Senshin-Iryo application submission to the Japanese Ministry of Health, Labour and Welfare.
“We are continuing the momentum of Edwards’ global transcatheter heart valve program with the first human implants in Japan. We look forward to providing this important treatment option to more Japanese patients and continuing to demonstrate very strong patient outcomes with the Edwards SAPIEN valve,” said Michael A. Mussallem, Edwards’ chairman and CEO.
The Edwards SAPIEN valve was approved for sale in Europe in late 2007. It has been used in the treatment of thousands of high-risk patients in both commercial and clinical trial environments, and evaluated as part of nine rigorous scientific studies now complete or underway. In the United States, it is being studied as part of the world’s only randomized, pivotal clinical trial of a transcatheter aortic valve.
For more information: www.edwards.com


 December 24, 2025
December 24, 2025 









