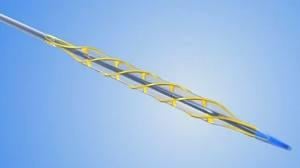
December 5, 2010 – Additional lots lots of the AngioSculpt Percutaneous Transluminal Angioplasty (PTA) Scoring Balloon Catheters are being recalled by the AngioScore Inc. The class I recall was originally initiated in December 2009, but the FDA reissued the alert in September. It was expanded this week to newer catheters produced between September 2007 and November 2010, which includes 17,682 units. The U.S. Food and Drug Administration (FDA) said a design defect may cause unintended fracture and peeling, which has resulted in peeling of the bond and/or detachment of the distal end of the scoring element. Use of affected devices may lead to retained device fragments or significant arterial injury, which may lead to death or need for additional surgical intervention. The new recall includes the AngioSculpt PTA Scoring Balloon Catheter, OTW 0.018" platform in multiple sizes. The following model part (REF) numbers and includes all sizes and lot codes for each model listed: • 2076-4020 • 2076-5020 • 2076-6020 • 2092-6020 • 2105-6020 AngioScore Inc. sent Medical Device Recall notification letters to all domestic customers on Nov. 15 by overnight mail. The recall letters list the affected product with description, part numbers and that all lots are affected. They describe the reason for recall and the potential harm to patients. The AngioSculpt PTA Scoring Balloon Catheter, OTW 0.018" platform is used for dilatation of lesions in the iliac, femoral, ilia-femoral, popliteal, infra-popliteal, and renal arteries, and for the treatment of obstructive lesions of native or synthetic arteriovenous dialysis fistulae. The original recall included the AngioSculpt EX PTCA scoring balloon catheters used to dilate narrowed coronary arteries and to improve myocardial perfusion. Those affected catheters included all part/REF numbers 2034-XXYY with lot numbers less than F09060003. Class 1 recalls are the most serious type of recall and involve situations in which there is a reasonable probability that use of these products will cause serious adverse health consequences or death. For more information, contact AngioScore customer service at 877.264.4692. For more information: www.fda.gov


 June 13, 2024
June 13, 2024 









