June 29, 2011 – An industry-first collaboration is bringing together cardiovascular testing company Atherotech Diagnostics Lab and diabetes testing company GlycoMark. The collaboration means the two companies will jointly commercialize the VAP (vertical auto profile) cholesterol test with the GlycoMark diabetes test.
The VAP test is the only expanded lipid test that routinely reports directly measured LDLc, which is included in the 22 reported cholesterol components — all at no additional cost. The VAP test also identifies markers of metabolic syndrome, often associated with early diabetes.
GlycoMark is a simple blood test used to measure after-meal glucose control in patients with diabetes. GlycoMark can accurately reveal potentially dangerous postprandial fluctuations in blood sugar — associated with the cardiovascular complications of diabetes — that are undetectable by other means, including the hemoglobin A1c (HbA1c) test.
“Just as standard lipid tests miss more than half of all patients at risk for heart disease, HbA1c misses dangerous glucose swings in about 40 percent of Type 2 diabetes patients,” said Michael E. Cobble, M.D., Atherotech Diagnostics Lab chief medical officer. “With diabetes now recognized as a major risk factor for cardiovascular disease, it’s time to recognize the value of well-validated clinical tests to more effectively monitor and treat these two disease states.”
The widely used HbA1c test measures average glucose levels over a two- to three-month period. GlycoMark provides a much more sensitive measure of variation over a one- to two-week period, thus indicating if blood glucose variability that comprises the average is minimal or extreme and whether treatment changes are indicated.
The GlycoMark test has proven particularly effective in patients with HbA1c values of less than eight percent — that is, those whose blood sugars were previously thought to be nearing or in control. GlycoMark is the only blood test proven to detect glycemic variability and hyperglycemic episodes in moderately controlled patients who have Type 1 or Type 2 diabetes mellitus, which affect more than 245 million adults and children worldwide.
“As a diabetologist, I routinely use GlycoMark to provide both the patient and me information we can't get from HbA1c alone,” said Irl B. Hirsch, M.D., professor of medicine at the University of Washington School of Medicine. “Patients appreciate the ability to better understand the ‘quality’ of their HbA1c.”
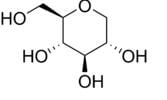
GlycoMark is a U.S. Food and Drug Administration (FDA)-approved test for monitoring intermediate glycemic control by measuring the levels of a monosaccharide 1,5-anhydroglucitol (1,5- AG) in blood. GlycoMark is being used in clinical practices nationwide and is now also available through Atherotech Diagnostics Lab. The test is also available at most major contract research organizations for pharmaceutical research studies.
Cholesterol components reported by the VAP test include Lp(a), apoB, apoA1, and the apoB/apoA1 ratio, making the VAP test the only lipid profile that routinely reports all three lipid parameters — LDL, non-HDL and apoB — considered necessary by the ADA and the American College of Cardiology. The two companies are co-exhibiting in GlycoMark’s booth #2133 at the 2011 American Diabetes Association (ADA) 71st Scientific Sessions June 24–28 in San Diego.
For more information: www.Atherotech, www.GlycoMark.com


 October 09, 2019
October 09, 2019 









