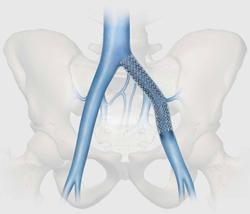
October 8, 2012 — Following Health Canada approval, Cook Medical made the Zilver Vena Venous Self-Expanding Stent available to physicians across Canada at the 2012 Annual Meeting of the Canadian Society for Vascular Surgery. Designed to restore blood flow in obstructed iliofemoral veins, Zilver Vena provides physicians with a tool designed specifically for stenting obstructed iliofemoral veins. This condition can arise for various reasons, including post-thrombotic syndrome in deep vein thrombosis (DVT) patients.
According to a study by Dr. A. Rosales of the department of vascular surgery at Oslo University Hospital in Norway, post-thrombotic syndrome (PTS) characterized by reflux and/or obstruction can be expected to develop in up to 40 percent of DVT patients.[1]
Built on Cook’s established line of Zilver stents, the Zilver Vena stent is a flexible, self-expanding stent made with “shape memory” nitinol. Zilver Vena was developed to address a challenging clinical issue, the need to establish and maintain blood flow in obstructed iliofemoral veins.
The stent provides flexibility, consistent radial force and continuous stent-to-vein wall apposition from end to end. Zilver Vena is currently available in 14 and 16 mm diameters and 60, 100 and 140 mm lengths to enable precise placement, and is compatible with 7 French sheaths and 9 French guiding catheters.
“As the only device of its kind available in Canada, Zilver Vena gives physicians a new treatment option for stenting diseased veins,” said Rob Lyles, vice president and global leader of Cook Medical’s Peripheral Intervention clinical division.
The Zilver Vena stent is currently under regulatory review by the U.S. Food and Drug Administration (FDA) and is not approved for sale in the United States.
For more information: www.cookmedical.com
Reference:
1. Rosales A. Sandbaek G, Jørgensen JJ. "Stenting for chronic post-thrombotic vena cava and iliofemoral venous occlusions: mid-term patency and clinical outcome." Eur J Vasc Endovasc Surg. 2010;40(2):234-240.


 November 24, 2025
November 24, 2025 









