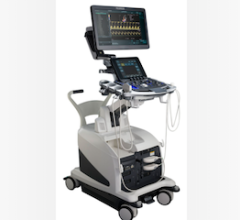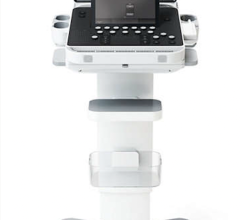
January 26, 2016 — Fujifilm SonoSite Inc. announced CE mark and U.S. Food and Drug Administration (FDA) 510(k) clearance for its new portable ultrasound system, the SonoSite Edge II. Designed with emergency medicine and critical care applications in mind, the Edge II features new transducer technology that delivers a better imaging experience for the most rugged environments.
In the acute care environment, reducing the time to make an accurate diagnosis is a critical need. The Edge II features DirectClear technology, a novel, patent-pending process that is available on select transducers. DirectClear elevates transducer performance by increasing penetration and contrast resolution. This transducer innovation, combined with a new wide-angle display that offers a 33 percent increase in viewing angles, contributes to an elevated imaging experience for the bedside clinician.
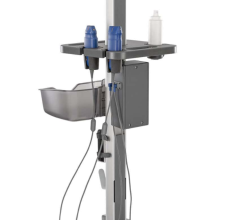
Designed to be truly portable and used in the most rugged environments, the Edge II incorporates Armored Cable Technology. With an embedded metal jacket, armored cables protect transducers from common abuse accident scenarios, like being rolled over, stepped on or twisted, and help maintain image quality over the life of transducers built on this cable platform.
For more information: www.sonosite.com

 October 09, 2025
October 09, 2025