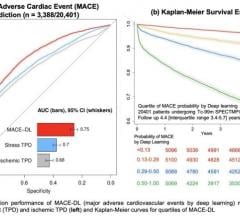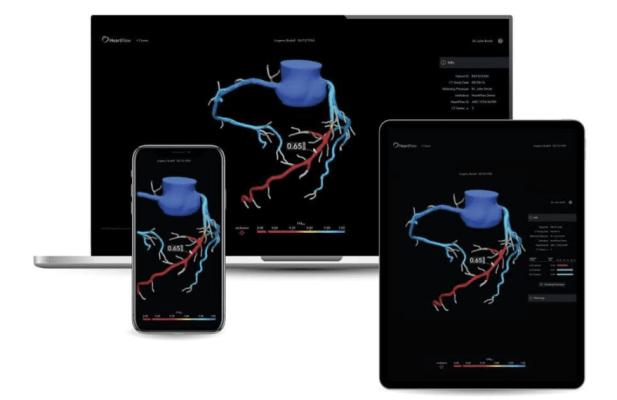
January 3, 2024 — HeartFlow, Inc., a leader in non-invasive artificial intelligence (AI) precision coronary care solutions, today announced that it has surpassed 250,000 patients receiving a HeartFlow FFRCT Analysis. This accomplishment is a testament to HeartFlow's dedication to driving a new standard of care and marks an important milestone for the company.
Traditional methods of non-invasive ischemia testing (stress EKG, stress echo, SPECT, PET, direct-to-cath) can result in false negatives 20-30 percent of the time, which can lead to undetected disease, and false positives over 50 percent of the time, which can lead to unnecessary invasive procedures.1,2 Together, Coronary Computed Tomography Angiography (CCTA) and HeartFlow’s FFRCT are part of a new standard of care that non-invasively diagnoses CAD and provides guidance on subsequent treatment decisions. HeartFlow’s FFRCT provides clear insights into each patient’s condition with a patient-specific visual model of the heart’s blood flow. With more than 500 peer-reviewed publications, the HeartFlow FFRCT Analysis remains unparalleled in precision coronary care, as supported by the ACC/AHA Chest Pain Guidelines, to improve treatment plans and outcomes.3 HeartFlow’s FFRCT Analysis is covered by Medicare and for >99% of commercially insured lives.
Since its inception, HeartFlow has been committed to building a new standard of care for people at risk of heart disease. For over a decade, HeartFlow’s deep partnerships with more than 1,000 hospitals globally, including 80% of the top 50 heart hospitals in the U.S., have advanced coronary care while also allowing customers to grow and scale their CCTA programs. HeartFlow is committed to serving customers quickly and reliably with a median turnaround time less than 1.5 hours.4 In addition, HeartFlow accepts ~95% of all CCTAs submitted by customers for an FFRCT analysis.5
To further underscore HeartFlow’s commitment to innovation, 2023 marked the release of its Plaque Analysis – the only FDA cleared plaque analysis with a reported 95% agreement compared to the gold standard, IVUS, in quantification and characterization of total plaque volume as shown in the REVEALPLAQUE study presented at the 2023 SCCT annual conference.6 Also in 2023, HeartFlow released its RoadMap Analysis which helps identify and quantify narrowings in the coronary arteries and has demonstrated a 25% faster CCTA read time.7
While HeartFlow was founded with FFRCT technology, the company has evolved from a single product used to assess the impact that narrowing vessels have on coronary blood flow (FFRCT Analysis), to a comprehensive suite of non-invasive technologies that build on CCTA. This FDA-cleared, comprehensive portfolio of technologies positions HeartFlow as the leader in the field of cardiac care - the first and most comprehensive AI-based decision tool empowering physicians in diagnosing and treating heart disease with unparalleled depth and precision.
“Accurate and timely diagnosis and treatment are fundamental to providing effective care, minimizing risks, preventing complications and improving the overall health for patients with CAD,” said Daniel I. Simon, MD, President, Academic & External Affairs and Chief Scientific Officer, University Hospitals Health System. “Our long-standing partnership with HeartFlow has directly contributed to enhanced diagnostic precision, improved turnaround times, a reduction in false positives and negatives, and a better patient experience. Complementing our clinical expertise, HeartFlow’s portfolio allows us to navigate the complex cardiac diagnostic and treatment process with enhanced visibility, greater confidence and the assurance that we are maximizing the potential for improved outcomes.”
“We are extremely proud of achieving the 250,000 patient milestone with our physician partners. Our collective team shares in our excitement for the future and are eager to build on this success.” said John Farquhar, Chief Executive Officer at HeartFlow. “We've experienced a year of notable accomplishments and growth including the expansion of our product portfolio with Plaque Analysis and RoadMap Analysis. Our continued commitment to developing a new standard of care for patients at risk of heart disease remains steadfast and we look forward to surpassing more milestones in the future.”
For more information: www.heartflow.com
References
1. Arbab-Zadeh, Heart Int 2012. Yokota, et al. Neth Heart J 2018. Nakanishi, et al. J Nucl Cardiol 2016.
2. Patel et al. NEJM 2010.
3. 2021 ACC/AHA Chest Pain Guidelines.
4. Turnaround time is defined as the time it takes from customer CCTA submission for HeartFlow to deliver back the FFRCT Analysis. US median turnaround time. Data on file.
5. Data on file.
6. REVEALPLAQUE Study, SCCT 2023.
7. Morris, et al. A Study to Measure the Ability of AI-CSQ to support the busy CCTA reader: SMART-CT. JCCT 2023.

 June 05, 2023
June 05, 2023