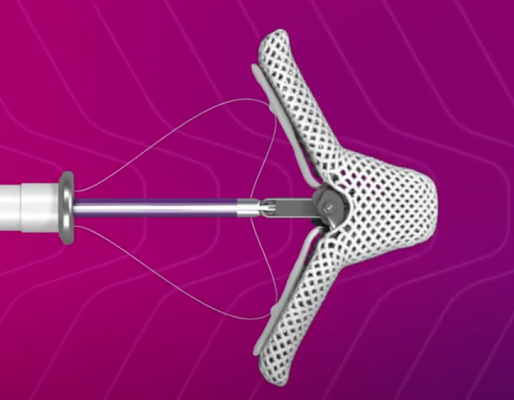
May 27, 2025 — Abbott has announced the U.S. Food and Drug Administration (FDA) has approved the company's Tendyne transcatheter mitral valve replacement (TMVR) system to treat people with mitral valve disease. This therapy is available for patients whose mitral valves are not functioning properly due to severe mitral annular calcification (MAC), a buildup of calcium within the ring-like structure (known as an annulus) that supports the mitral valve.
The complex nature of mitral valve disease and patients' specific needs and health conditions can pose challenges for surgical correction. For patients with severe MAC who are at high risk for open-heart surgery and whose mitral valve cannot be successfully repaired with the Abbott MitraClip device, Tendyne offers an alternative minimally invasive way to replace the valve that's leaky (mitral regurgitation) or narrowed (stenosis).
"Mitral annular calcification stiffens the structure of the mitral valve and can lead to mitral regurgitation or stenosis that disrupt the heart's ability to pump blood effectively. These conditions can have a significant impact on a patient's quality of life, causing symptoms such as chest pains, shortness of breath and dizziness," said Paul Sorajja, M.D., the Roger L. and Lynn C. Headrick Family Chair of the Valve Science Center for the Minneapolis Heart Institute Foundation and director of the Center for Valve and Structural Heart Disease for the Minneapolis Heart Institute at Abbott Northwestern Hospital. "Unfortunately, patients with MAC can be very difficult to operate on and many are considered too high risk for open-heart surgery due to multiple co-morbidities or other factors. Tendyne bridges a critical treatment gap for these patients and can help reduce the symptoms that can interfere with their lives."
The innovative and unique design of the Abbott Tendyne system and the valve's availability in multiple sizes allows it to adapt to a range of patient anatomies. The self-expanding valve is delivered through a small incision in the chest and then advanced into the heart to replace the mitral valve. The valve is fully repositionable and retrievable during implantation, allowing for the best possible outcome for people requiring a valve replacement.
"Tendyne is a much-needed addition to our comprehensive U.S. structural heart portfolio that offers less invasive treatment options for a range of heart diseases," said Sandra Lesenfants, senior vice president of Abbott's structural heart business. "This approval builds on our more than two decades of mitral valve leadership that includes developing first-of-their-kind devices that truly change – and save – people's lives."


 January 15, 2026
January 15, 2026 









