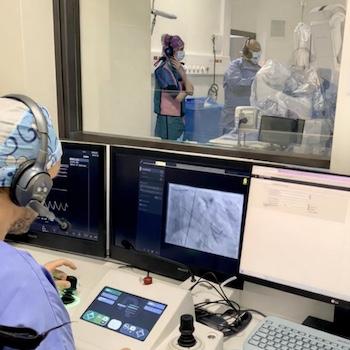
Photo: Robocath
Jan. 27, 2026 — Robocath has launched the world’s first FIH (First-In-Human) clinical study evaluating its new robotic system in coronary artery disease. This second-generation robot, developed thanks to the clinical and technological experience Robocath gained with its first robotic platform – which is already deployed in numerous centers worldwide – integrates unprecedented and unrivaled capabilities designed to meet the increasing demands of complex coronary procedures.
The study will recruit 20 patients with coronary artery disease, primarily complex cases representative of the routine activity of a catheterization laboratory. It will be conducted at the Centre Cardiologique du Nord (CCN) in Saint-Denis, France, under the leadership of Dr. Mohammed Nejjari, principal investigator, and Dr. Franck Digne, co-investigator.
The study will also benefit from the involvement of distinguished members of Robocath’s Medical Advisory Board (MAB), including Dr. Michael Haude and Dr. Jean Fajadet, who will provide their clinical and scientific expertise throughout the program.
“The launch of this First-In-Human study represents a major milestone for Robocath. With our second-generation robot we are leveraging the strong clinical and industrial experience acquired with our first platform, now in use internationally. This world first illustrates our leadership position and our commitment to continuing to push the boundaries of interventional robotic technology,” said Philippe Bencteux, president of Robocath.
This clinical launch confirms Robocath’s ambition to further strengthen its global leadership in this market and to contribute sustainably to the evolution of standards of care for patients with coronary artery disease.
Robocath is based in Rouen, France. Additional information is available at www.robocath.com.

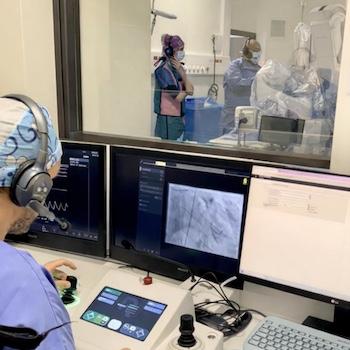

 April 26, 2024
April 26, 2024 





