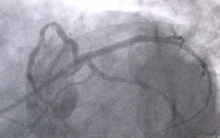
July 12, 2013 — Micell Technologies Inc. received CE mark approval for its MiStent sirolimus-eluting absorbable polymer coronary stent system. The MiStent SES is unique in providing local drug delivery both during and after the period of polymer absorption, thereby eliminating long-term polymer exposure, a potential cause of delayed healing and late adverse events.
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now