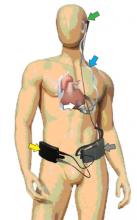
University Hospitals Case Medical Center and Case Western Reserve University School of Medicine (CWRU) researchers have uncovered a genetic factor that controls arrhythmias, the primary cause of sudden cardiac death (SCD) in the United States. The study will be published in the upcoming March edition of Nature entitled, “Circadian rhythms govern cardiac repolarization and arrhythmogenesis.”
© Copyright Wainscot Media. All Rights Reserved.
Subscribe Now