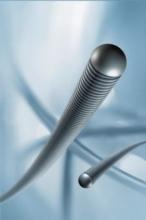
July 11, 2008 - AngioScore Inc. today announced the launch of new longer and larger AngioSculpt PTA Scoring Balloon Catheters for the treatment of peripheral artery disease (PAD).
The new devices have FDA 510(k) clearance to market for the dilatation of lesions in the iliac, femoral, ilio-femoral, popliteal, and infra popliteal arteries, and for the treatment of obstructive lesions of native or synthetic arteriovenous dialysis fistulae. The PTA catheter is not labeled for use in the coronary or neuro-vasculature.