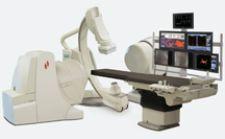
The Stereotaxis Navistar RMT Dual Sensor magnetically enabled 8mm ablation catheter has received FDA approval and will soon be commercially available in the U.S. The 8mm catheter expands electrophysiology applications for the Stereotaxis Magnetic Navigation system by providing physicians the ability to deliver high power ablations for the treatment of atrial arrhythmias.
Atrial flutter, a common atrial arrhythmia estimated to represent 25 percent of the over 400,000 ablation procedures performed worldwide each year, is routinely treated with 8mm catheters. Successful European cases have been completed at San Raffaele University Hospital in Milan, Italy and the Hanseatic Heart Center/St. Georg, Hamburg, Germany.


 October 28, 2025
October 28, 2025 









