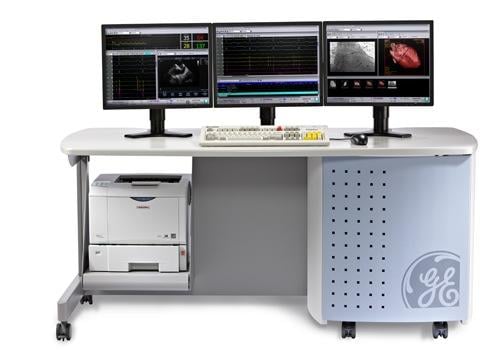
GE's CardioLab is one half of the integrated system with Abbott that will provide patient-specific data on the heart's electrical activity in real time.
May 13, 2015 — Abbott and GE Healthcare announced an agreement that will bring real-time, patient-specific data about the heart's electrical activity to cardiac electrophysiology labs. The goal of the collaboration is to speed up the diagnosis of the sources of atrial fibrillation and other heart rhythm disorders.
The integration of electrogram signal data from GE's CardioLab electrophysiology recording system into Abbott's RhythmView mapping software will allow doctors to use instantaneous electrical data of a person's heart to identify the sources of atrial fibrillation. By providing patient-specific diagnostics based on each person's unique physiology, this integrated system transforms current treatment approaches, which primarily rely on a one-size-fits-all anatomical approach.
Abbott's mapping software, or rotor identification system, helps physicians identify and target the specific areas of a person's heart that are perpetuating atrial fibrillation, the most common heart rhythm disorder. Abbott entered the catheter-based electrophysiology market last year through the acquisition of Topera Inc.
GE Healthcare’s CardioLab helps simplify the electrophysiology practice and streamline the workflow in diagnosis and treatment of complex cardiac cases.
For more information: www.abbott.com


 February 06, 2026
February 06, 2026 









