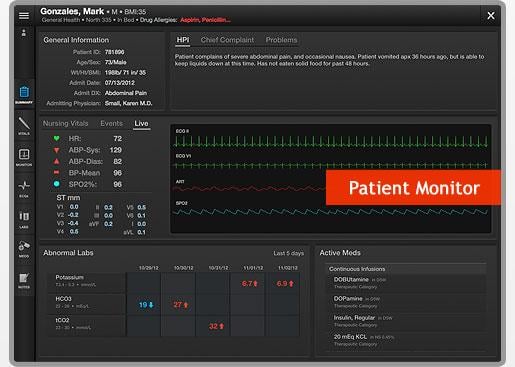
July 8, 2013 — AirStrip announced the first phase of its strategic participation in Microsoft’s AppsForSurface program. As part of the program, the company will launch and make available AirStrip ONE Cardiology not only on Windows 8.1-enabled mobile devices, but also on laptops and desktops.
“This program highlights Microsoft’s commitment to supporting the mHealth space with solutions that are proven to drive clinical transformation,” AirStrip CEO Alan Portela noted. “Thanks to this program, AirStrip will give healthcare providers a seamless user experience regardless of whether they are using AirStrip solutions on their Windows-enabled mobile devices, a Surface tablet, a laptop, or a desktop. The look, feel and depth of user experience will remain consistent for clinicians, regardless of the device they use. We are proud that Microsoft has recognized AirStrip ONE Cardiology as a pioneering mobile clinical application in the AppsForSurface program,” Portela added.
Coinciding with the Microsoft announcement, AirStrip is finalizing plans for expanded functionality for AirStrip ONE Cardiology that will support mobility throughout the entire cardiology care continuum. Portela indicated that a formal announcement detailing these added features will be released in the near future.
The announcement marks the latest in a series of firsts for AirStrip’s patent-protected and U.S. Food and Drug Administration (FDA)-cleared healthcare mobility solutions. Last month, AirStrip became the first and only mHealth solution for medical device mobility certified by the United States Air Force to comply with the Department of Defense Information Assurance Certification and Accreditation Process (DIACAP) security requirements. In February, AirStrip launched AirStrip ONE — the first enterprise-wide, data- and vendor-agnostic mobility solution to securely deliver patient data from medical devices, electronic medical records (EMRs) and patient monitors to clinicians anywhere across the care continuum, and announced that Dignity Health is AirStrip’s first customer for the AirStrip ONE solution.
For more information: www.airstrip.com


 January 15, 2026
January 15, 2026 









