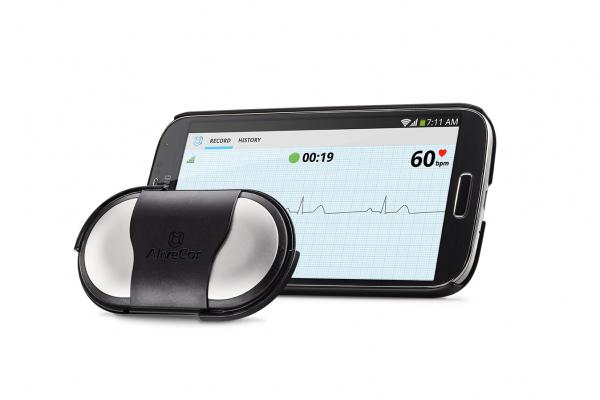
Image courtesy of AliveCor Inc.
February 2, 2015 — AliveCor Inc. announced it has received CE Mark clearance for its automated analysis process (algorithm) to detect atrial fibrillation (AF). The latest version of the AliveECG app for users in the United Kingdom and Ireland now provides patients with real-time AF detection in electrocardiogram (ECG) recordings using the AliveCor Heart Monitor. The AF Detector analyzes each ECG recorded and automatically notifies the user if AF is detected in that ECG, a step forward for anyone concerned with their own heart health or that of their family members.
Atrial fibrillation continues to be the most common heart rhythm disturbance, affecting about 2 million people in the UK and Ireland, and is responsible for a third of all strokes. AF and AF-related illness costs the National Health Service over £2.2 billion annually – a cost that is expected to rise as the incidence of AF accelerates. AF can be hard to detect, as symptoms, including heart palpitations, may be mild or non-existent. The AliveCor AF Detector allows patients to instantly know if AF is present in their ECG, and immediately take action as recommended by their physician.
The AliveECG app, currently free to customers, also includes features that help patients and physicians manage existing conditions with intelligent, personalized and easy-to-use new features. Tracking of medications, lifestyle choices and ongoing symptoms allows patients to have a better understanding of their health status. Advanced search features and enhanced graphical trends provide a more comprehensive and reliable view of a person's overall health.
"The new AF Detector, along with the tracking of medications and lifestyle factors will make it easier for me to identify and manage serious heart arrhythmias in my patients," said Richard Bogle, FACC, consultant cardiologist, St. Helier Hospital. "My patients will significantly benefit from being able to find out if they are having an AF episode and together it will allow us to determine which medications, habits and activities may be impacting their heart. With a more complete view of what is happening with my patients, even between appointments, we can make better decisions."
The AliveCor Heart Monitor is intended to record, store and transfer single-channel ECG rhythms. The device also displays ECG rhythms and detects the presence of atrial fibrillation. It is intended for use by healthcare professionals, patients with known or suspected heart conditions and health conscious individuals. The monitor is compatible with all iOS and most Android OS mobile devices. With secure storage in the cloud, users have the ability to access their data confidentially anytime, anywhere.
For more information: www.alivecor.com


 January 15, 2026
January 15, 2026 









