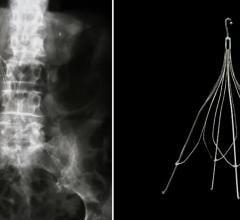
The Option Inferior Vena Cava Filter. Reprinted with permission of Angiotech Pharmaceuticals.
June 8, 2009 - Angiotech Pharmaceuticals Inc. today said the FDA granted 510(k) clearance for the Option Inferior Vena Cava (IVC) Filter for use in both permanent and retrievable indications.
Angiotech holds exclusive worldwide rights to market and distribute the Option IVC Filter, which it obtained in a license agreement with privately held Rex Medical LP.
The Option IVC Filter is used for the prevention of recurrent pulmonary embolism (PE). The device is implanted, typically by interventional radiologists in a minimally invasive procedure, into the body's inferior vena cava to prevent PE. Option is specifically designed for use as both a permanent or temporary implant.
Filter’s clinical study data shows a 92 percent retrieval success, and a retrieval at up to 175 days post-implantation.
According to market analysis conducted by Millennium Research Group, the U.S. market for IVC filters was approximately $200 million in 2007 (with approximately 160,000 IVC filters implanted). This market is predicted to grow to $300 million by 2012. IVC filters can be permanent or retrievable (where a physician can remove them when the patients no longer require them). Retrievable filters accounted for approximately two-thirds of the market in 2007. The Option IVC Filter is designed to be used in both permanent and retrievable indications and has been successfully retrieved at long intervals - up to 175 days post-implantation in the U.S. clinical trial.
The results of a recently concluded clinical trial for the Option IVC Filter were presented by the study's Principle Investigator Matthew Johnson, M.D., at the 34th Annual Scientific Meeting of the Society of Interventional Radiology in March of 2009. The single-arm, multicenter clinical trial, which enrolled 100 patients with a mean age of 59 years, was designed to evaluate the safety and efficacy of the Option IVC filter when used both as a permanent and temporary filter in patients at increased risk for pulmonary embolism. In the trial, clinical success, defined as placement technical success without subsequent PE, significant filter migration or embolization, symptomatic thrombosis or other complications requiring filter removal or intervention, was achieved in 88 percent of subjects. Retrieval success was achieved in 92 percent (36/39) of cases where retrieval was attempted, with a mean implantation time in those cases of 67 days. The safety profile of the Option IVC Filter was consistent with other currently marketed IVC filters.
For more information:www.angiotech.com


 July 27, 2021
July 27, 2021