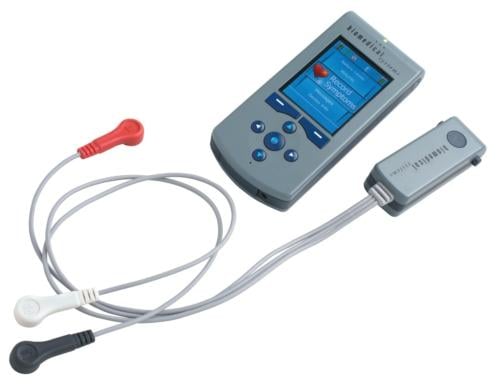
March 19, 2012 - Biomedical Systems, a global provider of cardiac diagnostic services and products, has introduced the TruVue Wireless Ambulatory ECG Monitoring System for the diagnosis and management of atrial fibrillation and other complex cardiac arrhythmia. TruVue's diagnostic benefits include the ability to record and wirelessly transmit every heartbeat for up to 30 days, perform advanced arrhythmia analysis and to provide immediate online access to all transmitted ECG.
TruVue offers electrophysiologists and cardiologists the advantages of both a Holter and cardiac event monitor in one device. Biomedical Systems developed the TruVue system in response to physicians' need for a long-term ECG monitoring device to diagnose symptoms suggestive of cardiac arrhythmia as well as manage the treatment of patients with atrial fibrillation. The patented technology employs cellular communication to transmit recordings of every heartbeat to Biomedical Systems' secure servers. The system's algorithms analyze the incoming data for changes in morphology, rate or rhythm, in addition to patient-triggered alerts when symptoms are experienced. Certified cardiac technicians validate the findings, post reports in real-time and alert physicians based on cardiac events or notification criteria. At any time, physicians can access information online, including any portion of the recorded ECG monitoring and trend reports detailing 24-hour heart-rate and -rhythm abnormalities, including atrial fibrillation.
"TruVue expedites the diagnoses of patients who may have cardiac arrhythmia, including atrial fibrillation, by delivering the most comprehensive long-term view of the heart rhythm," said Jim Ott, Biomedical Systems' cardiology division president. "With TruVue, we provide physicians with the unique ability to review every heartbeat for up to 30 days—combining both algorithm-triggered ECG-recordings and episodes recorded by patients—and immediately access information online without working through support staff."
TruVue has received both its 510(k) clearance from the U.S. Food and Drug Administration and its CE Mark for international sales. Biomedical Systems' Cardiology Division has employed TruVue since 2010, conducting thousands of ECG wireless telemetry procedures.
For more information: www.biomedsys.com


 January 15, 2026
January 15, 2026 









