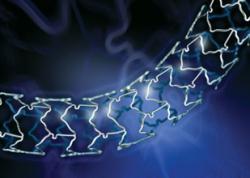
May 25, 2011 – Boston Scientific Corp. today announced it received approval from the U.S. Food and Drug Administration (FDA) to market its 2.25 mm Promus everolimus-eluting coronary stent system for use in vessels as small as 2.25 mm in diameter. The company plans to immediately launch the product in the United States. The Promus stent features a thin-strut, open-cell design to allow for excellent flexibility and conformability in the vessel. The low-profile stent and catheter tip help enhance deliverability, especially in small vessels. The Promus stent is supported by the SPIRIT clinical trial program, which demonstrates that the controlled release of everolimus results in low levels of late loss and a strong safety profile. The addition of this stent expands the available size matrix of Boston Scientific's Promus stent portfolio to include diameters from 2.25 to 4 mm and lengths from 8 to 28 mm. "The Promus stent has demonstrated outstanding deliverability, low late loss and excellent safety in numerous clinical trials and extensive real-world practice," said Dean J. Kereiakes, M.D., FACC, medical director, The Christ Hospital Heart and Vascular Center and The Carl and Edyth Lindner Center for Research and Education at The Christ Hospital, and professor of clinical medicine, Ohio State University. "These benefits make the Promus 2.25 mm stent an attractive option for U.S. physicians treating patients with small vessels." Data from clinical studies have shown that an estimated 10 percent of patients undergoing percutaneous coronary interventions have small vessels (less than 2.5 mm). The 2.25 mm Promus stent joins the 2.25 mm Ion paclitaxel-eluting platinum chromium stent as the company's approved drug-eluting stenting options for small vessels. The Promus stent is a private-labeled Xience V everolimus-eluting coronary stent system manufactured by Abbott and distributed by Boston Scientific. The stent is sold under the Promus name as part of a 2006 agreement from when Abbott purchased the Xience stent technology from Boston. For more information: www.bostonscientific.com


 November 24, 2025
November 24, 2025 









