January 24, 2014 — Boston Scientific has received U.S. Food and Drug Administration (FDA) clearance and CE mark approval for its Direxion Torqueable Microcatheter.
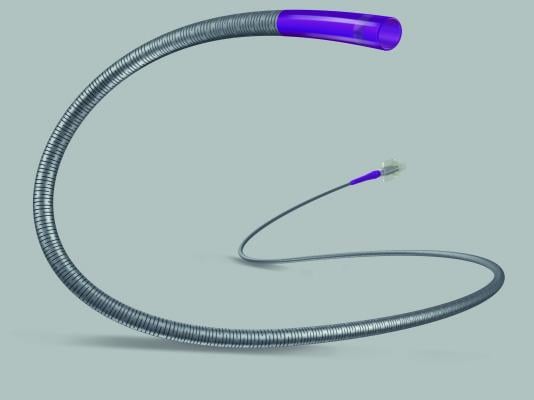
The Direxion Torqueable Microcatheter is designed to facilitate selective access and delivery of diagnostic, embolic and therapeutic materials into the peripheral vasculature. The
catheter, offered in either a 0.021 or 0.027 in inner diameter microcatheter, features a slotted, nitinol hypotube technology. This technology is designed to maximize torque transmission in the catheter shaft. The Direxion Torqueable Microcatheter is available in six tip configurations as well as pre-loaded configurations designed to suit a range of peripheral
embolization procedures. These configurations include the Fathom-16 guidewire, Transend-14 guidewire or Transend-18
guidewire.
For more information: www.bostonscientific.com