
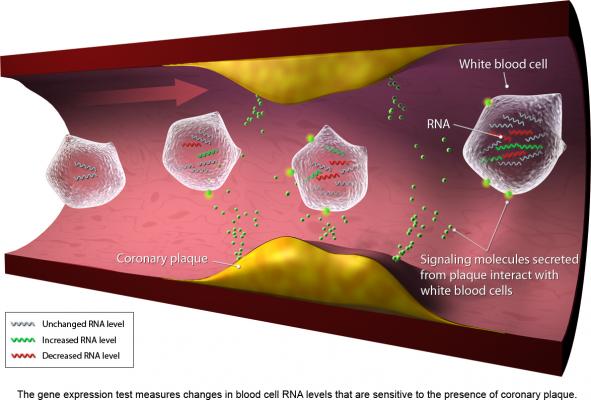
August 9, 2012 — CardioDx Inc. announced that Palmetto GBA, a national contractor that administers Medicare benefits, has established coverage for the company’s Corus CAD gene expression test for the evaluation of patients presenting with typical and atypical symptoms suggestive of coronary artery disease (CAD). With this decision, the Corus CAD gene expression test is now a covered benefit for more than 40 million Medicare enrollees in the United States.
With a simple blood draw, Corus CAD can safely, accurately and conveniently help primary care clinicians and cardiologists assess whether or not a stable non-diabetic patient’s symptoms are due to obstructive CAD, enabling many patients to avoid unnecessary invasive testing and exposure to imaging-related radiation risks or imaging agent intolerance. The test has been clinically validated in multiple independent patient cohorts, including two prospective, multicenter U.S. trials, PREDICT and COMPASS. Additionally, a retrospective, multicenter chart review study and the prospective IMPACT trial at Vanderbilt University demonstrated that Corus CAD use yielded significant and clinically relevant changes in patient management decisions in both primary care and cardiology settings.
Studies have shown that typical and atypical presentations of stable chest pain account for up to 2 percent of outpatient office visits each year in the United States, but as many as 62 percent of stable patients who undergo elective invasive angiographic procedures are found to have no obstructive coronary artery blockage, despite broad usage of prior noninvasive imaging. The authors of a 2010 New England Journal of Medicine study of nearly 400,000 coronary angiography patients concluded that current modalities used to identify patients for elective invasive angiography to diagnose obstructive CAD have limitations, and that better methods are needed for patient risk stratification.
“Identifying those symptomatic patients without a coronary blockage who may be able to avoid imaging or invasive testing is a significant problem for physicians, involving up to 10,000 patients daily in the U.S.,” said David Levison, president and CEO of CardioDx. “By providing Medicare beneficiaries access to Corus CAD, this coverage decision enables patients to avoid unnecessary procedures and risks associated with cardiac imaging and elective invasive angiography, while helping payers address an area of significant health care spending.”
For more information: www.cardiodx.com


 October 09, 2019
October 09, 2019 









