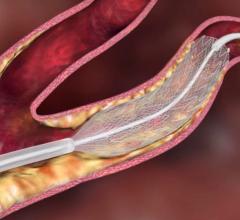
Cordis Corp. introduced next generation carotid stent system, the PRECISE PRO RX Nitinol Self-Expanding Stent, designed to treat clogged neck arteries and enable more efficient manipulation of the catheter and guidewire during stenting procedures and better crossability.
The PRECISE PRO RX Stent provides physicians with a broader range of treatment options for patients with carotid artery disease. It is the lowest profile system approved in the U.S. and among the lowest available in Europe. The PRECISE Stent is a small, wire-mesh tube that props open the blocked artery and may be used in conjunction with Cordis' ANGIOGUARD Rx Emboli Capture Guidewire System- a tiny, basket-like device designed to trap particles of plaque, blood clot, or other material that may be dislodged in the carotid artery during stent placement.
The PRECISE PRO RX Stent System is FDA and CE Mark approved to treat carotid artery disease in patients at high risk for adverse events from carotid endarterectomy (CEA) - a surgical treatment for removing arterial plaque from the carotid artery. The Cordis PRECISE Stent is the only carotid system backed by a large, randomized clinical trial - the landmark SAPPHIRE study - to support the potential benefits of carotid artery stenting (CAS) in patients who are ineligible, or considered high-risk, for carotid endarterectomy.

 June 26, 2023
June 26, 2023