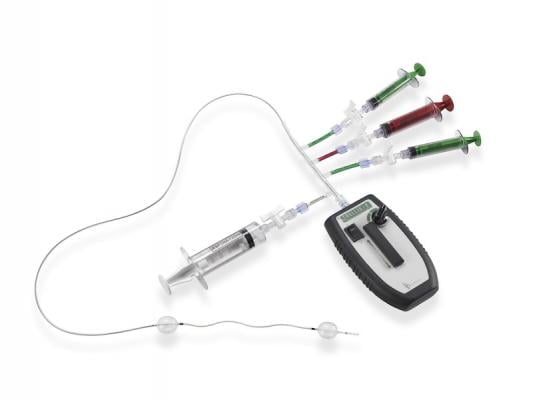
July 30, 2014 — Covidien announced the commercial launch of its next-generation Trellis peripheral infusion system. The redesigned system continues to be the only pharmacomechanical thrombolysis device that enables focused treatment of blood clots that lead to post-thrombotic syndrome (PTS). This latest Trellis system has been optimized to enhance drug delivery and the removal of the dissolved clot.
PTS is a long-term effect of deep vein thrombosis (DVT), a condition where a clot develops in one of the body’s deep veins above the knee. PTS develops when a DVT interferes with the blood flow returning from the leg back to the heart. It can cause lower leg pain and swelling, and can damage the valves in the veins that normally stop blood from flowing backwards down the leg. PTS occurs in almost half of patients within two years after DVT, and up to 33 percent of patients with PTS develop ulcers and skin deterioration.
The Trellis system provides a way for physicians to dissolve acute thrombus and intervene on DVT before it advances to PTS. The system is comprised of an over-the-wire catheter with two occlusive balloons to close off the treatment area and block drug release to other areas of the body; an infusion zone to deliver the lytic drug; and an oscillation drive unit that disperses the drug to dissolve the clot. The updated Trellis system features enhanced drug delivery and increased amplitude of the dispersion wire to better distribute the drug throughout the clot. Additionally, the new system features a larger aspiration window than the previous version, which allows for better removal of the drug and the dissolved clot.
“Covidien is committed to providing clinicians and patients with advanced technology that is clinically relevant and demonstrates economic value. Our clinical data has demonstrated that the Trellis system’s targeted lytic delivery may reduce the risk of bleeding complications normally associated with systemic thrombolytic infusion,” said Mark A. Turco, M.D., chief medical officer, vascular therapies, Covidien. “Furthermore, intervening in patients with DVT may help improve long-term outcomes in a population that can suffer significant complications if left untreated.”
The Trellis peripheral infusion system is currently available in the United States, Europe and Canada.
For more information: www.covidien.com


 October 28, 2025
October 28, 2025 









