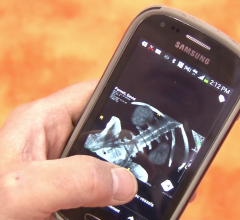
May 31, 2016 — MIM Software Inc. announced it has received 510(k) U.S. Food and Drug Administration (FDA) clearance to market full MIM products running on tablets through thin client technology such as Citrix.
This is the ninth 510(k) clearance MIM has received since 2000. To obtain clearance, MIM demonstrated that accessing its imaging software application via a thin client was equivalent to using the software on a traditional workstation. Retrieving imaging software on a thin client improves patient care by giving physicians flexibility in their workflow.
The 510(k) clearance applies to virtually all markets MIM serves including radiation oncology and nuclear medicine. As a result, it improves the decision-making process of physicians and users can be confident their interpretations will not be affected by mobile technology.
MIM will be demonstrating this technology at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) 2016 Annual Meeting, June 11–15 in San Diego.
For more information: www.mimsoftware.com

 April 05, 2019
April 05, 2019