The U.S. Food and Drug Administration (FDA) has granted approval of Medtronic’s premarket approval application (PMA) for the Melody Transcatheter Pulmonary Valve (TPV) and its Ensemble Transcatheter Valve Delivery System. This allows wider use of the valve in patients born with congenital heart defects that often require multiple open-heart surgeries. The valve is designed to reduce the number of open surgeries and expand the time between them.
The FDA said patients born with heart defects often receive a pulmonary valve conduit (an artificial graft with a valve inside that connects the heart to the lungs) to correct the defects. Over time, the conduit may become narrowed or leaky and will need to be replaced. The Melody TPV is used to treat a failing conduit.
Patients born with heart defects typically need several surgeries during their lifetime to correct their heart problems. The use of the Melody TPV will delay the time when a patient needs additional open-heart surgery. It can also reduce the total number of open-heart surgeries a patient needs.
The device is indicated for use as an adjunct to surgery in the management of pediatric and adult patients with the following clinical conditions:
• Existence of a full (circumferential) right ventricular outflow tract (RVOT) conduit that was equal to or greater than 16 mm in diameter when originally implanted; and
• Dysfunctional RVOT conduit with a clinical indication for intervention.
- more than moderate regurgitation and/or
- stenosis with a mean RVOT gradient of more than 35 mmHg
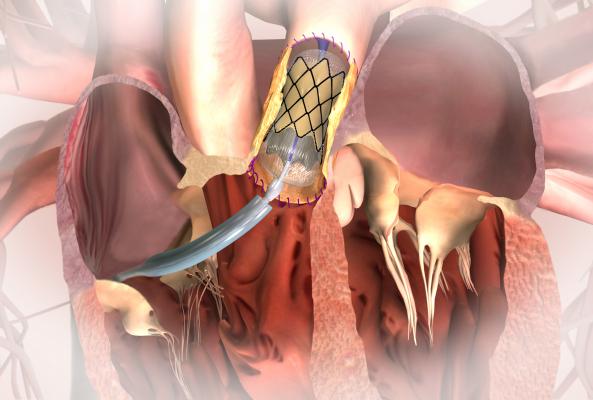
The Melody valve is made from the jugular vein valve of a cow that is sewn into a small metal frame. The Ensemble delivery system uses a balloon catheter to deploy the valve in the heart without open-heart surgery while the heart is beating. The device is first compressed onto the balloon at the tip of the delivery catheter for expansion in the native valve. The system uses femoral vein access.
The FDA said patients who receive the device will be followed up to five years to study the long-term effects and safety of the device. The OSB PAS - Long-term follow-up study is a prospective, non-randomized, multi-center, historically controlled clinical trial, designed to assess the postmarket performance of the Melody TPV in a representative population of providers and patients, with 5-year follow-up. The primary endpoint is freedom from TPV dysfunction, with a performance goal of 75 percent or greater at six months. Secondary endpoints include procedural success, serious procedural- and device-related adverse events, stent fracture, re-intervention on the TPV, surgical replacement of the RVOT conduit, death (all-cause, procedure-related and device-related), and New York Heart Association (NYHA) classification.
For more information: www.accessdata.fda.gov/cdrh_docs/pdf14/P140017a.pdf



 December 24, 2025
December 24, 2025 









