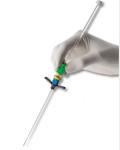
February 1, 2010 – The FDA granted market clearance for the PICC WAND introducer catheter, which enables clinicians to insert a peelable sheath for PICC or midline catheter placement using the new Accelerated Seldinger Technique (AST), for faster vascular access. The device is an all-in-one safety introducer that addresses the over-wire vascular access market by providing faster, safer and simpler vascular access. The PICC WAND combines all components of the older, Modified Seldinger Technique (MST) – the needle, guide wire, dilator and sheath – into one device that also protects against accidental needle sticks. Additionally, its “Fast-flash” feature provides early detection of vessel entry. Access Scientific partnered with Teleflex Medical to introduce the PICC WAND. It is designed to reduce the risk of accidental needle sticks, bleeding, contamination, guide wire embolism, and loss of cannulation. Air embolism is among the patient risks of MST that are reduced by the WAND and its Accelerated Seldinger Technique. Beginning in October 2008, the Centers for Medicare Medicaid Services (CMS) ceased reimbursing healthcare institutions for air embolism, which CMS considers preventable. The average cost to treat this complication, which can be fatal, is estimated at $66,000 per case. "The WAND facilitates PICC placement by enabling me to quickly and safely gain venous access," said Erin Capo, P.A. a vascular access specialist in the New York City metropolitan area. "The innovative design minimizes blood loss, decreases procedure time, optimizes operator safety and increases patient comfort. I have seen these benefits first-hand, and I am convinced that many other vascular specialists will adopt this technology." The device is designed to be used primarily by vascular nurse specialists for insertion of PICCs. The Seldinger technique was developed in 1953 to reduce complications associated with the introduction of catheters and other medical devices into blood vessels and hollow organs. Because there have been few significant improvements to the technique since it was invented, what is now known as the Modified Seldinger Technique (MST) still carries risks for patients and clinicians. For more information: www.The-Wand.com


 October 28, 2025
October 28, 2025 









