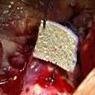
The TachoSil patch being used during an aortic valve replacement.
April 5, 2010 – The U.S. Food and Drug Administration (FDA) today said it approved the first absorbable fibrin sealant patch for use in cardiovascular surgery to prevent mild and moderate bleeding from small blood vessels, when standard surgical techniques are ineffective or impractical.
The TachoSil is a ready-to-use surgical patch composed of a dry collagen sponge made from horse tendons, and coated with fibrinogen and thrombin. At the site of a wound, the two proteins, through a series of chemical reactions, produce fibrin, a stringy, white, insoluble protein that allows a clot to form.
The patch is biodegradable and breaks down inside the body within four to six months. TachoSil is not intended for use within blood vessels.
“This approval provides an additional tool for surgeons to help control mild and moderate bleeding from blood vessels during cardiovascular surgery when standard surgical techniques are ineffective or impractical,” said Karen Midthun, M.D., acting director of the FDA’s Center for Biologics Evaluation and Research.
The plasma used to manufacture TachoSil is collected from U.S. donors who have been screened and tested for diseases transmitted by blood. The fibrinogen and thrombin used in the surgical patch undergo additional manufacturing processes to remove impurities, including blood-borne viruses. The collagen taken from horse tendons undergoes a separate step to remove impurities, including equine viruses.
The effectiveness of TachoSil manufactured by Nycomed Austria GmbH of Linz, Austria, was evaluated in a study of 119 cardiovascular surgery patients. Nearly three-quarters (74.6 percent) of those who received TachoSil, stopped bleeding within three minutes compared with 33.3 percent in the control group.
Hypersensitivity to product components or allergic reactions may occur with TachoSil. The adverse reaction rates were not statistically different between the study and control groups.
For more information: www.tachosil.com


 February 06, 2026
February 06, 2026 









