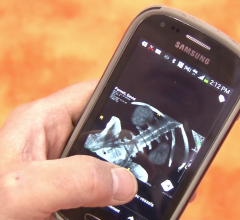
February 18, 2014 — The U.S. Food and Drug Administration (FDA) cleared Carestream Health’s Vue Motion image viewer for clinical viewing and reading of medical X-ray exams using iPhone 4s, iPad 2, Galaxy Note and Galaxy S III mobile devices.
Carestream’s development of zero footprint technology complements the “Bring Your Own Device” enterprise strategy that enables flexible data access by end users. Combined with DICOM including native 3-D server-side rendering as well as non-DICOM display, Vue Motion could be a universal clinical viewer.
Carestream recommends that during diagnostic image review, frequent and concurrent usage of Vue Motion viewing tools (such as zoom, pan and window level adjustments) be used. These tools help maximize image contrast and detail sharpness of each image displayed using Vue Motion on portable and mobile devices. Caution should be taken while viewing computed radiography (CR) and digital radiography (DR) images to ensure optimal contrast and detail sharpness.
Carestream experts will discuss Carestream’s Vue portfolio of healthcare IT solutions at HIMSS 2014, Feb. 24-27 at the Orlando Convention Center, Orlando, Fla.
For more information: www.carestream.com

 April 05, 2019
April 05, 2019